

Lactobacillus rhamnosus GG is classified as a strain from the Lactobacillus rhamnosus species. Lactobacillus rhamnosus GG is often referred to as Lactobacillus rhamnosus LGG® depending on probiotic manufacturer, however it should be noted that the strains are genetically identical.
L. rhamnosus GG is a very well-known and well researched probiotic. It has been included in over 250 clinical trials (approximately 40 in children) and according to clinicaltrials.gov, it is the most researched probiotic strain worldwide (Dronkers, Ouwehand and Rijkers, 2020).
As of April 2020, L. rhamnosus has been officially reclassified as Lacticaseibacillus rhamnosus, so the full strain name may also be referred to as Lacticaseibacillus rhamnosus GG (Zheng J et al., 2020).
Researched in: pre-term infants, newborns, children, adults and the elderly
L. rhamnosus GG has been demonstrated to reach the gut alive in numerous studies, even when a lower dosage of 100 million CFU was used (Petschow et al., 2005; Szachta, Ignyś and Cichy, 2011; DebMandal, Mandal and Pal, 2012). L. rhamnosus GG has also been extensively trialled in thousands of individuals from pre-term infants to the elderly for over 30 years and has demonstrated an excellent safety profile (Capurso, 2019). The strain is widely used in probiotics supplements and is extremely well tolerated with few, if any, significant side effects.
A retrospective study analysed data from two NICU’s in Italy from 2003-2008. Very low birth weight infants (VLBW) admitted to the NICU’s were given three billion CFU of L. rhamnosus GG from the 4th day of life for a four to six week period (average birth weight 1056g, gestational age 29.5 weeks). Infants received expressed mothers’ milk with pre-term formula supplementation if required. Data was analysed from 743 infants over the five year period, no adverse effects were noted and L. rhamnosus GG was not associated with any sepsis cases. The researchers concluded the probiotic to be safe and well tolerated (Manzoni et al., 2011). A similar study in VLBW infants also found L. rhamnosus GG alongside lactoferrin to be safe and well tolerated, plus it significantly reduced the incidence of necrotising enterocolitis (NEC) (Manzoni et al., 2014).
It should be noted that one trial did find bacteraemia associated with L. rhamnosus GG in one infant with short gut syndrome indicating that further care and research is needed for probiotic supplementation in infants with this condition (De Groote et al., 2005).

Upper respiratory tract infections (URTI’s) are one of the most common infections in both adults and children. Children typically experience five to six episodes per year and those at school may even get up to 12 per year (Toivonen et al., 2016). It is estimated that children attending day care have approximately 2-3 times greater the risk of developing RTI’s (Lu et al., 2004). Probiotics have been extensively trialled for their ability to reduce URTI’s, a recent Cochrane review additionally found certain strains to be effective in reducing the number of participants experiencing episodes of acute URTI, the mean duration of an episode of acute URTI, antibiotic use and cold-related school absence (Hao, Dong and Wu, 2015). L. rhamnosus GG was included in the Cochrane review and has also been favourably assessed in meta-analyses as a single strain.
A meta-analysis conducted in 2013 assessed L. rhamnosus GG for its ability to reduce the risk of respiratory infections in children. Four randomised controlled trials comprising 1,805 children were included, which met the researcher’s inclusion criteria. L. rhamnosus GG significantly reduced the incidence of URTI’s, otitis media infections and antibiotic use in children. Additionally, subgroup analysis found L. rhamnosus GG was able to significantly reduce overall respiratory tract infections in children over one year of age (S. Liu et al., 2013).
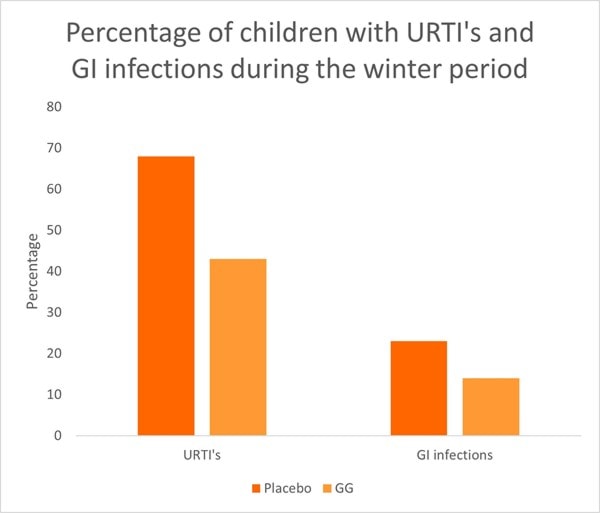
A key study from the meta-analysis was a RCT conducted in Croatia. The study assessed 281 children, aged one to seven years old, across day care centres. Children were divided into groups to receive either a fermented milk drink supplemented with one billion CFU of L. rhamnosus GG or the milk drink alone without supplementation (control). Children took their respective milk drink daily for three months over the winter period. In the probiotic group, only 43% of children developed an URTI compared with 68% in the placebo group (p<0.001) (Graph 1). There was also a reduction in gastrointestinal infections in the probiotic group reaching near significance (p<0.08).
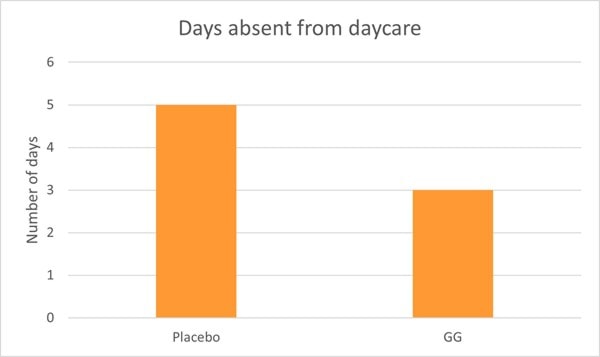
Children in the probiotic group also experienced significantly shorter bouts of illness and less absence from day care compared to the control group (Graph 2) (Hojsak, Snovak, et al., 2010). Other studies have also confirmed the reduction in URTI’s in children attending day care centres in Finland (Hatakka, 2001; Kumpu et al., 2012).
Hospital acquired infections are common in children and can result in additional medication and longer stays. Researchers conducted a RCT to investigate whether L. rhamnosus GG was able to reduce the occurrence of nosocomial gastrointestinal and respiratory tract infections in a paediatric hospital. The RCT included 742 children (average age=10) who were randomised to receive either a fermented milk containing one billion CFU of L. rhamnosus GG or the same milk without probiotic (control). Children received their daily milk c g in the morning from the day they were admitted until discharge. RTI’s and GI infections were significantly lower in the probiotic group than the control group. Only 2% of children in the probiotic group developed RTI’s compared with 5.5% in the control group. Additionally, those in the probiotic group had a reduced risk of developing an RTI that lasted longer than three days (Hojsak, Abdović, et al., 2010). Another RCT also found L. rhamnosus GG (6 billion CFU) alongside B vitamins, Vitamin C and zinc was able to significantly reduce the incidence of hospital acquired infections compared with controls (9% v 33%), as well as having significantly shorter stays in hospital (Bruzzese et al., 2016).
A recent RCT investigated L. rhamnosus GG in malnourished children (in terms of incidence of infection and anthropometric and metabolic parameters). The study included 71 children aged between six months to five years. The children were divided into two groups; probiotic and control. All children received an appropriate calorie and protein diet and the probiotic group additionally received one billion CFU of L. rhamnosus GG per day. Several parameters were assessed once a month for a total of three months. In the probiotic group, URTI’s, acute gastroenteritis and total infection incidence rates were significantly lower every month and at the end of the study compared to the control group and baseline values. By month three, 0.24 URTI episodes were noted in the probiotic compared with 1.47 episodes at baseline. Additionally, in the probiotic group, the number of UTI’s were significantly lower than the control group by the end of the study.
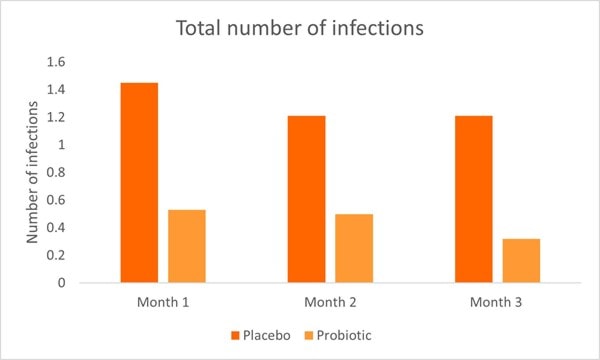
Hospitalisation and total number of infections were significantly higher in the control group (Graph 3) compared to the probiotic group by the end of the study. Overall the study demonstrated the positive effects of L. rhamnosus GG on URTI’s, acute gastroenteritis, UTI’s and overall infection rates (Kara, Volkan and Erten, 2019).
An RCT in pre-term infants found L. rhamnosus GG (given from day 3-60) significantly lowered the incidence of RTI’s, especially RTI’s caused by rhinoviruses (Luoto et al., 2014).
Cow's milk protein allergy (CMPA) is one of the most common food allergies seen in children, affecting approximately 2-7.5% of infants under one year (Ludman, Shah and Fox, 2013). Typically, formula-fed babies will be prescribed with an extensively hydrolysed formula (EHF) or amino acid formula. Breastfeeding mothers with a CMPA infant will eliminate dairy from their diet. Dairy is often reintroduced around the age of one to check tolerance, however, it can take children until age five to outgrow the allergy. CMPA can be challenging with variations in symptom severity. L. rhamnosus GG has been researched in infants with CMPA and shown to be safe and well tolerated (Scalabrin et al., 2009, 2017).
An RCT analysed L. rhamnosus GG in children aged 0-12 years diagnosed with CMPA. All infants were placed on a cow's milk free diet; formula fed infants received EHF and mothers who were breastfeeding cut dairy from their diet. Children were then divided into two groups; probiotic (milk free diet plus one billion CFU/day of L. rhamnosus GG) or control (milk free diet alone). After four weeks of intervention, researchers assessed symptoms associated with CMPA. In the control group, bloody stool, restiveness, and abdominal distension significantly improved from baseline. However, in the probiotic group, there was also a significant reduction in bloody stool, restiveness, abdominal distension as well as diarrhoea, mucus in stool and vomiting. There was also a general reduction in dermatitis in the probiotic group (p=0.07). The study highlights that L. rhamnosus GG can further improve CMPA symptoms beyond standard milk changes alone (Basturk et al., 2020).
L. rhamnosus GG has also been assessed in other studies and found to significantly improve digestive symptoms associated with CMPA (Baldassarre et al., 2010; Guest and Fuller, 2019). One study additionally found significantly reduced faecal calprotectin levels with L. rhamnosus GG supplementation (Baldassarre et al., 2010).
As well as symptom improvement, L. rhamnosus GG supplementation has been shown to improve the rate of oral tolerance. A prospective multi-centre study assessed different diets on the rate of tolerance in children with CMPA. The study assessed 256 healthy CMPA infants aged between 1 to 12 months. All participants had received a standard formula 15-30 days prior to recruitment of this study and had no CMPA symptoms. Children received either 1) extensively hydrolysed casein formula (EHCF)(n=55), 2) EHCF and L. rhamnosus GG (n=71), 3) hydrolysed rice formula (HRF)(n=46), 4) soy formula (n=55) and 5) amino acid based formula (n=33). At the start of the study, all infants underwent anamnestic and clinical evaluation, skin prick tests, atopy patch tests and an oral food challenge to confirm CMPA diagnosis. After one year, a food challenge test was conducted again to assess acquisition of tolerance (Graph 4).
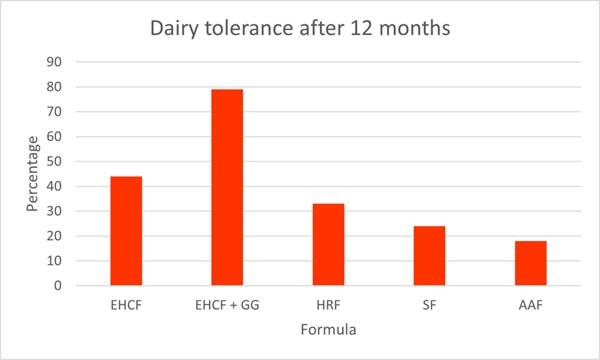
The highest rate of tolerance was noted in group 2; EHCF and L. rhamnosus GG, where 78.9% of children had acquired tolerance by one year (p<0.05). EHCF also had significantly higher levels of tolerance (43.6%) compared to the other groups; HRF(32.6%), soy formula (23.6%), and amino acid based formula (18.2%). These results clearly demonstrate the augmented effect with the addition of L. rhamnosus GG (Berni Canani et al., 2013).
A later study by the same researcher also found the addition of L. rhamnosus GG to EHCF further increased the rate of oral tolerance in IgE mediated CMPA infants and reduces the incidence of other allergic manifestations (Berni Canani et al., 2017).
A study in 2004 may explain how L. rhamnosus GG is able to improve symptoms and tolerance associated with CMPA. The study found children with CMPA often have a deficiency in IFN-gamma response and that L. rhamnosus GG is able to raise IFN-gamma through a peripheral blood mononuclear cell (PBMC) modulation (Pohjavuori et al., 2004).
Clinical trials in colicky infants can be challenging due to its varying aetiologies. However, several studies have documented altered microbiome profiles (Dubois and Gregory, 2016; Rhoads et al., 2018; Ouald Chaib et al., 2020). Typically, colicky babies have exhibited higher levels of gas producing and inflammatory microbes and a reduction in diversity as well as lower levels of beneficial Bifidobacteria. Probiotics may therefore play a role in the management of colic.
An RCT assessed L. rhamnosus GG on crying and irritability in 94 pre-term infants (gestational age 32-36 weeks). Infants were randomised to receive either a prebiotic mixture (GOS and polydextrose), probiotic (L. rhamnosus GG) or a placebo between one to three days old. Newborns took their respective supplement daily for two months. In the probiotic group, infants received 1 billion CFU for the first month and 2 billion CFU during the second month. In the placebo group, 47% of babies exhibited excessive crying compared with 19% in both pre and probiotic groups (Graph 5).
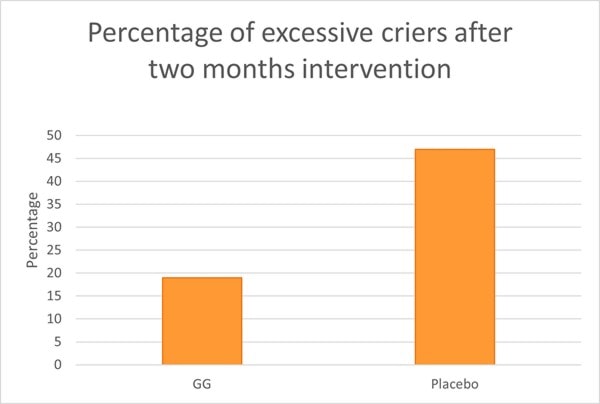
Interestingly Bifidobacteria levels did not differ between contented and excessive criers, however, the composition of Bifidobacterium spp. was different. B. infantis levels were significantly lower in excessive criers compared with contented babies. Clostridium histolyticum type bacteria were found to be significantly lower in the probiotic group compared with prebiotic and placebo. These microbes are well known for their toxin producing abilities. Overall L. rhamnosus GG was able to reduce the risk of excessive crying and have modulatory effects on the newborn's gut microbiome (Pärtty et al., 2013) .
Another RCT investigated L. rhamnosus GG and its potential to reduce crying time in 30 children with colic. All children were placed on a cow’s milk free diet (either EHCF or milk exclusion via breastfeeding) and then divided into two groups; probiotic and placebo. The probiotic group received 4.5 billion CFU of L. rhamnosus GG. After four weeks, parents reported a 68% reduction in daily crying compared with 49% in the placebo (p=0.05). The number of cry days per week was also lower in the probiotic group. Additionally, in the probiotic group, increased Bifidobacteria species were noted. Bifidobacterium lactis increased in 47% of infants compared with 13% in the placebo and Bifidobacterium breve counts increased in 40% of infants compared with 13% in the placebo. This study highlights potential for L. rhamnosus GG in colicky babies (Pärtty, Lehtonen, et al., 2015)
Diarrhoea in children can lead to dehydration and severe complications. Despite this, there is little over the counter medication they can take to prevent diarrhoea or to manage early symptoms. L. rhamnosus GG has been found to alleviate diarrhoeal symptoms in both GI infections and antibiotic associated diarrhoea (AAD).
The Centres for Disease Control and Prevention (CDC) state that diarrhoeal diseases account for 1 in 9 child deaths worldwide, making diarrhoea the second leading cause of death among children under the age of 5 (Liu et al., 2012). Hospital acquired diarrhoea is also prevalent, it is estimated 5-44% of hospitalised children develop infections, typically GI or RTI’s. L. rhamnosus GG has already been shown to significantly reduce the risk of hospital acquired infections, specifically GI infections (Hojsak, Snovak, et al., 2010; Bruzzese et al., 2016). L. rhamnosus GG has also been used in numerous studies to treat diarrhoea.
A recent meta-analysis assessed the efficacy of L. rhamnosus GG to treat acute diarrhoea (Li et al., 2019). The researchers included 19 RCTs (n=4073). Administration of L. rhamnosus GG was found to significantly reduce the duration of diarrhoea and stay in hospital. Time taken to improved stool consistency was also quicker when L. rhamnosus GG was used. The risk of diarrhoea lasting more than three to four days was significantly lower in the probiotic groups than placebo. L. rhamnosus GG also had beneficial effects in children with diagnosed rotavirus infections, one of the most common agents of diarrhoeal infections. It is thought that L. rhamnosus GG is able to support the epithelial lining by modulating dendritic cell and inflammatory cytokines, as well as elicit anti-viral effects (F. Liu et al., 2013; Jiang et al., 2017). Overall, the researchers found L. rhamnosus GG to be an effective treatment option for diarrhoea, especially when used in dosages of over 10 billion CFU and advised early L. rhamnosus GG administration.
A large RCT included in the meta-analysis assessed children (aged 6 months-5 years) with acute watery diarrhoea. Children were randomised to receive either a five day probiotic protocol (10 billion CFU of L. rhamnosus GG per day) or no probiotic intervention. All children also received standard WHO management treatment for diarrhoea. The duration of diarrhoea was significantly shorter in the probiotic group than control (60hr vs 78hr). A faster improvement in stool consistency was also noted in the probiotic group (Aggarwal et al., 2014).
A 2021 meta-analysis of 84 studies involving 13,443 children (aged 18 and under) found that certain single-strain probiotics, including L. rhamnosus GG, effectively treated acute diarrhea in children with various certainty evidence. (Zengbin L, et al., 2021)
Antibiotics are the most prescribed medication given to children (Nicolini and Sperotto, 2014) and respiratory tract infections are responsible for 74.4% of antibiotic prescriptions in children worldwide (O’Brien et al., 2015). Antibiotics given in a child’s early years can have current and long-lasting effects on their health and wellbeing. Diarrhoea is one of the most common side effects of antibiotic use.
A meta-analysis assessed the efficacy of L. rhamnosus GG to prevent AAD in adults and children. The review included 12 RCT’s (n=1499), five of which were specifically in children (n=445). L. rhamnosus GG significantly reduced the risk of developing AAD in patients from 22.4% to 12.3%. Interestingly, further analysis revealed the risk reduction was only significant in children, not adults. A range of antibiotics were used in the trials, indicating the type of antibiotic does not impact the efficacy of L. rhamnosus GG (Szajewska and Kołodziej, 2015).
One of the standout RCT’s from the meta-analysis was a large trial including 188 children aged 6 months to 10 years who were prescribed a 10 day antibiotic course. Children were divided into groups to receive either 10-20 billion CFU of L. rhamnosus GG (depending upon weight) or a placebo alongside their prescription. Only 8% of participants in the probiotic group developed AAD, compared with 26% in the placebo. The duration of diarrhoea was also significantly less in the probiotic group and stool consistency improved significantly quicker in the probiotic group compared with placebo (Vanderhoof et al., 1999)
A more recent RCT also assessed L. rhamnosus GG as a preventative for the development of AAD in 90 boys (aged 13-36 months) undergoing hypospadias repair. The patients were divided into three groups to receive either 1) antibiotic and L. rhamnosus GG 2) antibiotic alone and 3) antibiotic and placebo. Participants took their respective supplement daily from the day of surgery until the end of hospitalisation. The incidence of AAD was significantly lower in the probiotic group compared with group 2 and 3 (10% v 50% and 40% respectively). The duration of AAD was also significantly lower with L. rhamnosus GG. Interestingly, the frequency of dressing change and the incidence of postoperative complications (urethral fistula and foreskin dehiscence) were also significantly lower in the probiotic group (Esposito et al., 2018).
In the UK and worldwide it is very well accepted that digestive symptoms and IBS can significantly impact children and their quality of life and schooling. A recent study in the US found approximately 24.7% of children aged 0-3 years and 25% of children aged 4-18 years met the Rome IV criteria for a functional GI disorder (Robin et al., 2018).
Another RCT also assessed L. rhamnosus GG in 141 children with IBS or functional abdominal pain. Children were divided to receive either 6 billion CFU of L. rhamnosus GG or a placebo for eight weeks. After a further eight week follow up, researchers found that children in the L. rhamnosus GG group had a significant reduction in pain severity and pain frequency. Additionally, treatment success was seen in 87% of children in the probiotic group compared with 50% of children in the placebo group (Graph 6).
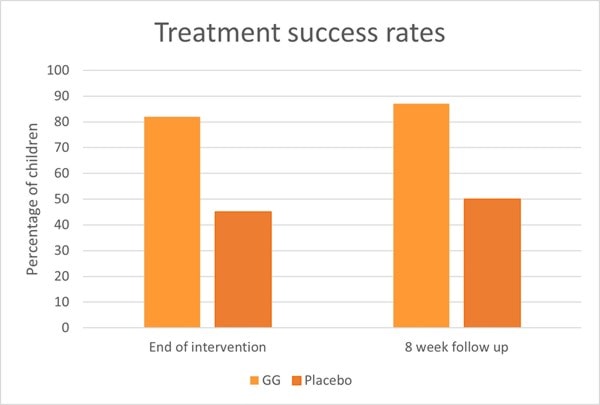
At baseline, 59% of children exhibited abnormal results from an intestinal permeability test, however in the probiotic group the number of children with abnormal results decreased after intervention (p< 0.03) (Francavilla et al., 2010). An explanation for the improved intestinal permeability could lie within in vitro studies. In vitro evidence has shown L. rhamnosus GG to stimulate intestinal epithelial cell proliferation and enhance secretion of protective mucins (Mack et al., 1999, 2003). These actions help support the integrity of the gut epithelium and additionally protect against the effects of pathogens, antibiotics and negative dietary factors.
An RCT included 104 children (average age 11) who fulfilled the Rome II criteria for IBS, functional dyspepsia (FD), or functional abdominal pain (FAP). Children received either 3 billion CFU of L. rhamnosus GG or a placebo daily for four weeks. After intervention, those receiving the probiotic were more likely to have treatment success than those in the placebo group (25% v 9.6% respectively). The results were more apparent in IBS, where treatment success was achieved in 33% of individuals compared with 5% in the placebo group (p<0.05). More children with IBS who received L. rhamnosus GG reported reduced frequency and severity of pain compared to the placebo group, although reduction in pain severity was not significant (p=0.01) (GAWROŃSKA et al., 2006)
An RCT assessed 52 children (aged 4-18) with abdominal pain. Participants were divided into two groups: probiotic and placebo. In the probiotic group, children received 10 billion CFU of L. rhamnosus GG per day for four weeks. After one week of intervention, abdominal pain had significantly decreased in the probiotic group. This was also significant at the end of week two, three and four. Additionally, there was a significant improvement in the functional scale after two weeks of treatment (Kianifar and Khalesi, 2015).
The relationship between the microbiome and atopic dermatitis (AD) is complex. The gut-skin axis is only recently starting to be truly explored, however early research is clearly demonstrating our gut microbiome composition plays a key role. Lower levels of Bifidobacteria and higher levels of Clostridia are consistently seen in children with AD (Thomas and Fernández-Peñas, 2017; Capurso, 2019). The research for L. rhamnosus GG and atopic dermatitis (eczema) is inconsistent. This could perhaps be explained by the complexity of AD development, family history, dietary and environmental factors and the timing of probiotic intervention as well as dosage.
One of the most promising studies gave L. rhamnosus GG prenatally to mothers (within the last 2-4 weeks of pregnancy) with a family history of atopy. The RCT divided 132 pregnant women into two groups to receive either 20 billion CFU of L. rhamnosus GG or a placebo daily until they gave birth. For 6 months post-delivery, breastfeeding mothers continued to take the probiotic and formula fed infants were given the capsule contents mixed with water via a spoon. Recovery rates of L. rhamnosus GG in breastfed and formula-fed infants stools did not differ significantly. Only 23% of children who received L. rhamnosus GG supplementation developed eczema compared with 46% in the placebo group (Kalliomäki et al., 2001).
A seven year follow up study was completed by 73% of the original participants. The study found the protective benefits of L. rhamnosus GG were sustained. The cumulative risk for developing eczema within the first seven years was significantly lower in the probiotic group than placebo (42.6% v 66.1% respectively) (Kalliomäki et al., 2007).
Attention deficit hyperactivity disorder (ADHD) is characterized by pervasive and impairing symptoms of inattention, hyperactivity and impulsivity and is thought to affect 5.29% of individuals worldwide (Polanczyk et al., 2007). There is currently a lack of research on the link between the gut microbiome and ADHD, however L. rhamnosus GG has shown early promise.
An RCT assessed whether L. rhamnosus GG may reduce the risk of ADHD in later childhood (Pärtty, Kalliomäki, et al., 2015). The trial followed up on participants from a previous study that was initiated 13 years prior (Kalliomäki et al., 2001). In brief, newborns received 20 billion CFU daily for the first six months of life. 76 of the original infants attended follow up and for assessment of neuropsychiatric disorders (including ADHD). By age 13, no children who had received L. rhamnosus GG developed ADHD or Aspergers, compared with 6 (17%) children in the placebo group (p= 0.008). Interestingly, those who had developed ADHD or Aspergers exhibited significantly lower levels of Bifidobacteria at six months old. However, by age 13, no significant microbial differences could be detected. It is well known that the first thousand days of life are one of the most important development stages and thus offers a window of opportunity to exert positive influences on later health. This study may offer potential insight into the development of some neuropsychiatric disorders and potentially, how to lower their risk.
An RCT included 32 children (aged 4-17) diagnosed with ADHD. They received either 10 billion CFU of L. rhamnosus GG or a placebo daily for three months. Those receiving the probiotic had significantly improved quality of life scores compared to baseline values (not significant in placebo) after the intervention period (Kumperscak et al., 2020). The authors hypothesised that L. rhamnosus GG was able to support gut integrity via its effect on tight junctions, mucin and IgA production. A mouse study found that L. rhamnosus GG was able to regulate emotional behaviour and the central GABAergic system via the vagus nerve (Bravo et al., 2011), which may support the results seen in Kumperscak et al.
Both studies highlight exciting potential for L. rhamnosus GG, however it should be emphasised that more studies are required to ascertain the probiotic impact.
Individuals with cystic fibrosis (CF) often display high levels of inflammation, mucus formation and microbial dysbiosis (Debyser et al., 2016). There is potential for the use of probiotics, especially given their ability to reduce RTI’s through the gut-lung axis. L. rhamnosus GG has been assessed in a few pilot studies in children with CF.
A controlled prospective study specifically assessed intestinal inflammation in 30 children with CF. A total of 75 children were included; 30 with CF, 30 healthy controls and 15 with active inflammatory bowel disease. Ten of the children with CF received 5 billion L. rhamnosus GG daily for four weeks. After intervention faecal calprotectin levels significantly decreased in this group. These results highlight the potential of L. rhamnosus GG to reduce gut inflammation in CF patients (Bruzzese et al., 2004). Reduced calprotectin levels were also found in a later trial using L. rhamnosus GG, the study also found distinct microbial changes in CF individuals (Bruzzese et al., 2014).
A pilot RCT included 38 children with CF who were divided into two groups. Group 1 received 6 billion CFU per day of L. rhamnosus GG for six months, followed by a four week wash out period and then placed on oral rehydration solution (ORS) for an additional six months. Group 2 reversed the order and started with 6 months ORS, a four week wash out period and then six months taking 6 billion CFU of L. rhamnosus GG per day. L. rhamnosus GG administration resulted in a significant reduction in pulmonary exacerbations (p=0.0035) and of hospital admissions (p=0.001) compared to ORS treatment. The probiotic also resulted in greater FEV1 scores (Bruzzese et al., 2007).
Surprisingly a larger recent study in 2018, found L. rhamnosus GG did not have beneficial effects in children with CF. The RCT divided 95 children to receive either 6 billion CFU of L. rhamnosus GG or a placebo daily for 12 months. No differences in pulmonary exacerbations or hospital admissions were noted. The authors concluded that perhaps the trial was conducted under more stringent conditions or whether a child’s age may play a role in L. rhamnosus GG efficacy (Bruzzese et al., 2018).
Overall the ambiguous results highlight further research is required to determine the effects of L. rhamnosus GG in children with CF.
L. rhamnosus GG has been extensively researched in children and with childhood health conditions. However, it is also a highly researched strain in adults and has shown numerous benefits.
Information on this strain was gathered by Dr Kate Stephens PhD Food and Microbial Sciences; Gut Microbiology (University of Reading), BSc Medical Microbiology.
Last updated – January 2022.
As some properties & benefits of probiotics may be strain-specific, this database provides even more detailed information at strain level. Read more about the strains that we have included from this genus below.
Lactobacillus acidophilus strains: Lactobacillus acidophilus LA-05, Lactobacillus acidophilus NCFM®, Lactobacillus acidophilus Rosell-52.
Lactobacillus casei strains: Lactobacillus casei Shirota, Lactobacillus casei DN-114001.
Lactobacillus plantarum strains: Lactobacillus plantarum LP299v.
Lactobacillus reuteri strains: Lactobacillus reuteri Protectis and Lactobacillus reuteri RC-14®.
Lactobacillus rhamnosus strains: Lactobacillus rhamnosus GR-1® , Lactobacillus rhamnosus HN001 and Lactobacillus rhamnosus Rosell-11.
Lactobacillus paracasei strains: Lactobacillus paracasei CASEI 431®.
For more information and the latest research on probiotics, please visit the Probiotic Professionals pages.
Aggarwal, S. et al. (2014) ‘Lactobacillus GG for treatment of acute childhood diarrhoea: an open labelled, randomized controlled trial’, Indian J Med Res ., 139(3), pp. 379–385.
Baldassarre, M. E. et al. (2010) ‘Lactobacillus GG Improves Recovery in Infants with Blood in the Stools and Presumptive Allergic Colitis Compared with Extensively Hydrolyzed Formula Alone’, The Journal of Pediatrics, 156(3). doi: 10.1016/j.jpeds.2009.09.012.
Basturk, A. et al. (2020) ‘Investigation of the Efficacy of Lactobacillus rhamnosus GG in Infants With Cow’s Milk Protein Allergy: a Randomised Double-Blind Placebo-Controlled Trial’, Probiotics and Antimicrobial Proteins, 12(1). doi: 10.1007/s12602-019-9516-1.
Berni Canani, R. et al. (2013) ‘Formula Selection for Management of Children with Cow’s Milk Allergy Influences the Rate of Acquisition of Tolerance: A Prospective Multicenter Study’, The Journal of Pediatrics, 163(3). doi: 10.1016/j.jpeds.2013.03.008.
Berni Canani, R. et al. (2017) ‘Extensively hydrolyzed casein formula containing Lactobacillus rhamnosus GG reduces the occurrence of other allergic manifestations in children with cow’s milk allergy: 3-year randomized controlled trial’, Journal of Allergy and Clinical Immunology, 139(6). doi: 10.1016/j.jaci.2016.10.050.
Brahma S et al. (2022) Probiotics: A gut response to the COVID-19 pandemic but what does the evidence show? Clinical Nutrition ESPEN, 51: 17-27. doi.org/10.1016/j.clnesp.2022.08.023
Bravo, J. A. et al. (2011) ‘Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve’, Proceedings of the National Academy of Sciences, 108(38). doi: 10.1073/pnas.1102999108.
Bruzzese, E. et al. (2004) ‘Intestinal inflammation is a frequent feature of cystic fibrosis and is reduced by probiotic administration’, Alimentary Pharmacology & Therapeutics, 20(7). doi: 10.1111/j.1365-2036.2004.02174.x.
Bruzzese, E. et al. (2007) ‘Effect of Lactobacillus GG supplementation on pulmonary exacerbations in patients with cystic fibrosis: A pilot study’, Clinical Nutrition, 26(3). doi: 10.1016/j.clnu.2007.01.004.
Bruzzese, E. et al. (2014) ‘Disrupted Intestinal Microbiota and Intestinal Inflammation in Children with Cystic Fibrosis and Its Restoration with Lactobacillus GG: A Randomised Clinical Trial’, PLoS ONE, 9(2). doi: 10.1371/journal.pone.0087796.
Bruzzese, E. et al. (2016) ‘Randomised clinical trial: a Lactobacillus GG and micronutrient-containing mixture is effective in reducing nosocomial infections in children, vs. placebo’, Alimentary Pharmacology & Therapeutics, 44(6). doi: 10.1111/apt.13740.
Bruzzese, E. et al. (2018) ‘Lack of efficacy of Lactobacillus GG in reducing pulmonary exacerbations and hospital admissions in children with cystic fibrosis: A randomised placebo controlled trial’, Journal of Cystic Fibrosis, 17(3). doi: 10.1016/j.jcf.2017.10.014.
Capurso, L. (2019) ‘Thirty Years of Lactobacillus rhamnosus GG’, Journal of Clinical Gastroenterology, 53(Supplement 1). doi: 10.1097/MCG.0000000000001170.
DebMandal, M., Mandal, S. and Pal, N. K. (2012) ‘Detection of intestinal colonization of probiotic Lactobacillus rhamnosus by stool culture in modified selective media’, Asian Pacific Journal of Tropical Disease, 2(3). doi: 10.1016/S2222-1808(12)60047-4.
Debyser, G. et al. (2016) ‘Faecal proteomics: A tool to investigate dysbiosis and inflammation in patients with cystic fibrosis’, Journal of Cystic Fibrosis, 15(2). doi: 10.1016/j.jcf.2015.08.003.
Dronkers, T. M. G., Ouwehand, A. C. and Rijkers, G. T. (2020) ‘Global analysis of clinical trials with probiotics’, Heliyon, 6(7). doi: 10.1016/j.heliyon.2020.e04467.
Dubois, N. E. and Gregory, K. E. (2016) ‘Characterizing the Intestinal Microbiome in Infantile Colic’, Biological Research For Nursing, 18(3). doi: 10.1177/1099800415620840.
Esposito, C. et al. (2018) ‘Frequency of Antibiotic-Associated Diarrhea and Related Complications in Pediatric Patients Who Underwent Hypospadias Repair: a Comparative Study Using Probiotics vs Placebo’, Probiotics and Antimicrobial Proteins, 10(2). doi: 10.1007/s12602-017-9324-4.
Francavilla, R. et al. (2010) ‘A Randomized Controlled Trial of Lactobacillus GG in Children With Functional Abdominal Pain’, PEDIATRICS, 126(6). doi: 10.1542/peds.2010-0467.
GAWROŃSKA, A. et al. (2006) ‘A randomized double-blind placebo-controlled trial of Lactobacillus GG for abdominal pain disorders in children’, Alimentary Pharmacology & Therapeutics, 25(2). doi: 10.1111/j.1365-2036.2006.03175.x.
De Groote, M. A. et al. (2005) ‘LACTOBACILLUS RHAMNOSUS GG BACTEREMIA ASSOCIATED WITH PROBIOTIC USE IN A CHILD WITH SHORT GUT SYNDROME’, Pediatric Infectious Disease Journal, 24(3). doi: 10.1097/01.inf.0000154588.79356.e6.
Guest, J. F. and Fuller, G. W. (2019) ‘Effectiveness of using an extensively hydrolyzed casein formula supplemented with Lactobacillus rhamnosus GG compared with an extensively hydrolysed whey formula in managing cow’s milk protein allergic infants’, Journal of Comparative Effectiveness Research, 8(15). doi: 10.2217/cer-2019-0088.
Hao, Q., Dong, B. R. and Wu, T. (2015) ‘Probiotics for preventing acute upper respiratory tract infections’, Cochrane Database of Systematic Reviews. doi: 10.1002/14651858.CD006895.pub3.
Hatakka, K. (2001) ‘Effect of long term consumption of probiotic milk on infections in children attending day care centres: double blind, randomised’, BMJ, 322(7298). doi: 10.1136/bmj.322.7298.1327.
Hojsak, I., Snovak, N., et al. (2010) ‘Lactobacillus GG in the prevention of gastrointestinal and respiratory tract infections in children who attend day care centers: A randomized, double-blind, placebo-controlled trial’, Clinical Nutrition, 29(3). doi: 10.1016/j.clnu.2009.09.008.
Hojsak, I., Abdović, S., et al. (2010) ‘Lactobacillus GG in the Prevention of Nosocomial Gastrointestinal and Respiratory Tract Infections’, Pediatrics, 125(5). doi: 10.1542/peds.2009-2568.
Jiang, Y. et al. (2017) ‘Effects of Lactobacillus rhamnosus GG on the maturation and differentiation of dendritic cells in rotavirus-infected mice’, Beneficial Microbes, 8(4). doi: 10.3920/BM2016.0157.
Kalliomäki, M. et al. (2001) ‘Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial’, The Lancet, 357(9262). doi: 10.1016/S0140-6736(00)04259-8.
Kalliomäki, M. et al. (2007) ‘Probiotics during the first 7 years of life: A cumulative risk reduction of eczema in a randomized, placebo-controlled trial’, Journal of Allergy and Clinical Immunology, 119(4). doi: 10.1016/j.jaci.2006.12.608.
Kara, S. S., Volkan, B. and Erten, I. (2019) ‘Lactobacillus rhamnosus GG can protect malnourished children’, Beneficial Microbes, 10(3). doi: 10.3920/BM2018.0071.
Kianifar, H. and Khalesi, M. (2015) ‘Probiotic for irritable bowel syndrome in pediatric patients: a randomized controlled clinical trial’, Electron Physician, 7(5).
Kumperscak, H. G. et al. (2020) ‘A Pilot Randomized Control Trial With the Probiotic Strain Lactobacillus rhamnosus GG (LGG) in ADHD: Children and Adolescents Report Better Health-Related Quality of Life’, Frontiers in Psychiatry, 11. doi: 10.3389/fpsyt.2020.00181.
Kumpu, M. et al. (2012) ‘Milk containing probiotic Lactobacillus rhamnosus GG and respiratory illness in children: a randomized, double-blind, placebo-controlled trial’, European Journal of Clinical Nutrition, 66(9). doi: 10.1038/ejcn.2012.62.
Lebeer S et al., (2022) Selective targeting of skin pathobionts and inflammation with topically applied lactobacilli. Cell Reports Medicine, 3(2) DOI:https://doi.org/10.1016/j.xcrm.2022.100521
Li, Y.-T. et al. (2019) ‘Efficacy of Lactobacillus rhamnosus GG in treatment of acute pediatric diarrhea: A systematic review with meta-analysis’, World Journal of Gastroenterology, 25(33). doi: 10.3748/wjg.v25.i33.4999.
Liu, F. et al. (2013) ‘Lactobacillus rhamnosus GG on Rotavirus-Induced Injury of Ileal Epithelium in Gnotobiotic Pigs’, Journal of Pediatric Gastroenterology & Nutrition, 57(6). doi: 10.1097/MPG.0b013e3182a356e1.
Liu, L. et al. (2012) ‘Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000’, The Lancet, 379(9832). doi: 10.1016/S0140-6736(12)60560-1.
Liu, P. et al, 'Research Advances in the Treatment of Allergic Rhinitis by Probiotics', J Asthma Allergy. 2022;15:1413-1428.
Liu, S. et al. (2013) ‘Lactobacillus rhamnosus GG supplementation for preventing respiratory infections in children: A Meta-analysis of Randomized, Placebo-controlled Trials’, Indian Pediatrics, 50(4). doi: 10.1007/s13312-013-0123-z.
Lu, N. et al. (2004) ‘Child day care risks of common infectious diseases revisited’, Child: Care, Health and Development, 30(4). doi: 10.1111/j.1365-2214.2004.00411.x.
Ludman, S., Shah, N. and Fox, A. T. (2013) ‘Managing cows’ milk allergy in children’, BMJ, 347(sep16 1). doi: 10.1136/bmj.f5424.
Luoto, R. et al. (2014) ‘Prebiotic and probiotic supplementation prevents rhinovirus infections in preterm infants: A randomized, placebo-controlled trial’, Journal of Allergy and Clinical Immunology, 133(2). doi: 10.1016/j.jaci.2013.08.020.
Mack, D. R. et al. (1999) ‘Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression’, American Journal of Physiology-Gastrointestinal and Liver Physiology, 276(4). doi: 10.1152/ajpgi.1999.276.4.G941.
Mack, D. R. et al. (2003) ‘Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro’, Gut, 52(6). doi: 10.1136/gut.52.6.827.
Manzoni, P. et al. (2011) ‘Routine Lactobacillus rhamnosus GG administration in VLBW infants: A retrospective, 6-year cohort study’, Early Human Development, 87. doi: 10.1016/j.earlhumdev.2011.01.036.
Manzoni, P. et al. (2014) ‘Bovine lactoferrin supplementation for prevention of necrotizing enterocolitis in very-low-birth-weight neonates: a randomized clinical trial’, Early Human Development, 90. doi: 10.1016/S0378-3782(14)70020-9.
Nicolini, G. and Sperotto, F. (2014) ‘Combating the rise of antibiotic resistance in children’, Minerva Pediatrica, 66(1), pp. 31–39.
O’Brien, K. et al. (2015) ‘Clinical predictors of antibiotic prescribing for acutely ill children in primary care: an observational study’, British Journal of General Practice, 65(638). doi: 10.3399/bjgp15X686497.
Ouald Chaib, A. et al. (2020) ‘The influence of the gastrointestinal microbiome on infant colic’, Expert Review of Gastroenterology & Hepatology, 14(10). doi: 10.1080/17474124.2020.1791702.
Pärtty, A. et al. (2013) ‘Effects of Early Prebiotic and Probiotic Supplementation on Development of Gut Microbiota and Fussing and Crying in Preterm Infants: A Randomized, Double-Blind, Placebo-Controlled Trial’, The Journal of Pediatrics, 163(5). doi: 10.1016/j.jpeds.2013.05.035.
Pärtty, A., Kalliomäki, M., et al. (2015) ‘A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: a randomized trial’, Pediatric Research, 77(6). doi: 10.1038/pr.2015.51.
Pärtty, A., Lehtonen, L., et al. (2015) ‘Probiotic Lactobacillus rhamnosus GG therapy and microbiological programming in infantile colic: a randomized, controlled trial’, Pediatric Research, 78(4). doi: 10.1038/pr.2015.127.
Petschow, B. W. et al. (2005) ‘Effects of Feeding an Infant Formula Containing Lactobacillus GG on the Colonization of the Intestine’, Journal of Clinical Gastroenterology, 39(9). doi: 10.1097/01.mcg.0000177245.53753.86.
Pohjavuori, E. et al. (2004) ‘Lactobacillus GG effect in increasing IFN-γ production in infants with cow’s milk allergy’, Journal of Allergy and Clinical Immunology, 114(1). doi: 10.1016/j.jaci.2004.03.036.
Polanczyk, G. et al. (2007) ‘The Worldwide Prevalence of ADHD: A Systematic Review and Metaregression Analysis’, American Journal of Psychiatry, 164(6). doi: 10.1176/ajp.2007.164.6.942.
Rhoads, J. M. et al. (2018) ‘Infant Colic Represents Gut Inflammation and Dysbiosis’, The Journal of Pediatrics, 203. doi: 10.1016/j.jpeds.2018.07.042.
Robin, S. G. et al. (2018) ‘Prevalence of Pediatric Functional Gastrointestinal Disorders Utilizing the Rome IV Criteria’, The Journal of Pediatrics, 195, pp. 134–139. doi: 10.1016/j.jpeds.2017.12.012.
Scalabrin, D. et al. (2017) ‘Long-term safety assessment in children who received hydrolyzed protein formulas with Lactobacillus rhamnosus GG: a 5-year follow-up’, European Journal of Pediatrics, 176(2). doi: 10.1007/s00431-016-2825-4.
Scalabrin, D. M. et al. (2009) ‘Growth and Tolerance of Healthy Term Infants Receiving Hydrolyzed Infant Formulas Supplemented With Lactobacillus rhamnosus GG: Randomized, Double-Blind, Controlled Trial’, Clinical Pediatrics, 48(7). doi: 10.1177/0009922809332682.
Ser, H. et al. (2022) IDDF2022-ABS-0236 Healing the GUT with probiotics: can probiotics help relieve allergic rhinitis? Gut, 71:A63-A64.
Szachta, P., Ignyś, I. and Cichy, W. (2011) ‘An Evaluation of the Ability of the Probiotic Strain Lactobacillus rhamnosus GG to Eliminate the Gastrointestinal Carrier State of Vancomycin-resistant Enterococci in Colonized Children’, Journal of Clinical Gastroenterology, 45(10). doi: 10.1097/MCG.0b013e318227439f.
Szajewska, H. and Kołodziej, M. (2015) ‘Systematic review with meta-analysis: Lactobacillus rhamnosus GG in the prevention of antibiotic-associated diarrhoea in children and adults’, Alimentary Pharmacology & Therapeutics, 42(10). doi: 10.1111/apt.13404.
Thomas, C. L. and Fernández-Peñas, P. (2017) ‘The microbiome and atopic eczema: More than skin deep’, Australasian Journal of Dermatology, 58(1). doi: 10.1111/ajd.12435.
Toivonen, L. et al. (2016) ‘Burden of Recurrent Respiratory Tract Infections in Children’, Pediatric Infectious Disease Journal, 35(12). doi: 10.1097/INF.0000000000001304.
Vanderhoof, J. A. et al. (1999) ‘Lactobacillus GG in the prevention of antibiotic-associated diarrhea in children’, The Journal of Pediatrics, 135(5). doi: 10.1016/S0022-3476(99)70053-3.
Zengbin L et al., (2021) Which Probiotic Is the Most Effective for Treating Acute Diarrhea in Children? A Bayesian Network Meta-Analysis of Randomized Controlled Trials. Nutrients, 13: 4319.