Microbiology profile:
Origin: Healthy human infant
Key words: Probiotic, children, infants, newborn, immunity, gut health, bifidobacteria, colic
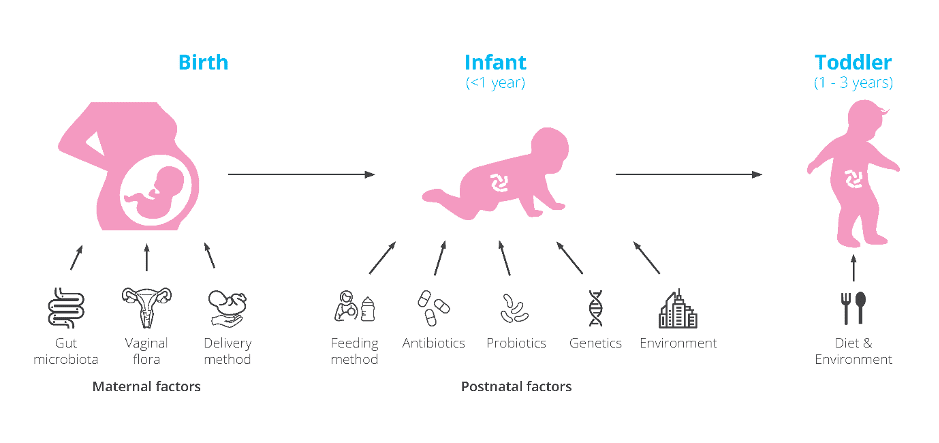
The first three years of life are essential for microbiome and immune system development. These early years can shape our future health. Factors such as delivery mode (C-section or vaginal birth), gestational age, formula or breast feeding, and antibiotic use (Figure 1) can impact our microbiome which can increase the risk of present and future health issues.
Bifidobacteria are a bacterial genus associated with immune regulation and digestive health. They are expected to dominate in a healthy infant’s gut. There are numerous studies highlighting their key role in infants for digestive support and immune modulation. Emerging research is additionally showing that the actual species of Bifidobacteria present is important. Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium bifidum and Bifidobacterium infantis should be present in high numbers (Wong B et al., 2019). Many strains belonging to these species can promote the barrier effect and competitively inhibit potential pathogens. These species also have the ability to break down human milk oligosaccharides (HMO’s), a highly selective prebiotic found in breast milk. During fermentation of HMO’s, they can produce short chain fatty acids which promote a healthy gut environment. In addition, these species may contain digestive enzymes such as ß galactosidases which help to break down lactose and other nutrients found in milk. (It should be noted that all of these abilities are strain specific). Good digestion and absorption of nutrients are essential in these early years due to infant growth and demanding energy requirements. This could explain the importance of Bifidobacteria and the microbiome in early years.
Bifidobacteria numbers are usually low in pre-term births, likely due to the many challenges these infants face from long stays in hospital, antibiotic use and possible enteral feeding. Typically, Bifidobacteria colonisation is delayed giving the opportunity for pathogenic bacteria to thrive with little competition in the gut. Enterobacteria and Streptococci are common colonisers in premature babies (Sakata H et al., 1985). This could explain why premature infants are at higher risks of infections, necrotising enterocolitis (NEC) or sepsis. Additionally, Bifidobacteria numbers may be lower in C-section babies which could explain why C-section babies are often at higher risk of asthma, allergies and metabolic diseases (Thavagnanam S et al., 2008).
It’s traditionally been thought that formula fed infants have lower numbers of Bifidobacteria, however recent research is showing that this may not be the case. Some studies have reported formula fed babies may have similar numbers of Bifidobacteria but it is not the dominant genus. There is proportionally less due to many other microbial genera being present, some potentially harmful (Lee SA et al., 2015). Additionally, the species of Bifidobacteria differ between breast fed and formula fed infants, with Bifidobacterium spp. in formula fed infants resembling species more commonly seen in adults (Turroni F et al., 2009). There is also some emerging research showing that Bifidobacteria species may also differ between C-section and vaginally born babies. It was found that Bifidobacteria colonisation occurred within three days of birth with B. bifidum and B. longum species dominating (Makino H., 2018) in vaginally born infants.
Colic commonly occurs in newborn’s. Colic has been associated with microbial imbalances, reduced diversity and inflammation. High levels of Escherichia spp. and Klebsiella spp. and low levels of Bifidobacteria have been associated with crying time in newborns (Dubois N et al., 2016) (De Weerth C et al., 2013). Therefore, rebalancing a baby’s microbiome by boosting levels of Bifidobacteria may reduce symptoms associated with colic.
This information provides researchers with a window of opportunity to intervene through the use of probiotics specifically formulated for an infant’s gut.
Bifidobacterium breve M-16V® has been demonstrated in clinical trials to be safe and can reach the gut alive. There have been numerous trials in preterm babies and those with necrotising enterocolitis (NEC) demonstrating the safety of the strain in vulnerable populations.
No adverse events were noted in the probiotic group during the trial period. B. breve counts significantly increased in stools of the probiotic group. This result gives strong evidence of the strain’s survival through the gastro-intestinal tract (Patole et al., 2014).
A baby biome should exhibit high levels of Bifidobacteria and lower numbers of Enterobacteria including Escherichia coli from birth. A delay in Bifidobacteria colonisation and dysbiosis may increase the risk of health conditions later in life.
High levels of Bifidobacteria may support overall health including digestion and immunity, but their numbers are also associated with happier temperaments (Aatsinki A et al., 2019). This may be part of the reason why a healthy microbiome may reduce the risk of colic.
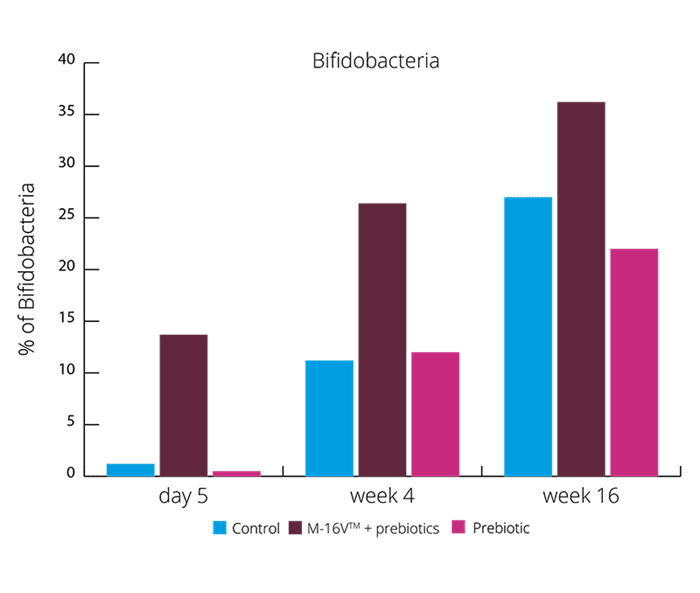
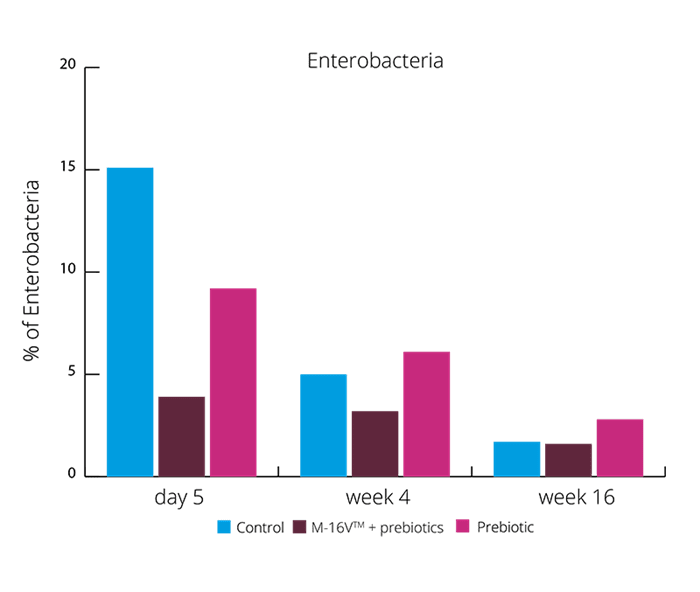
These results suggests the synbiotic group was able to create a more beneficial microbial balance. This is also supported by higher levels of acetate and lower faecal pH. (Chua MC et al., 2017). Increasing the levels of Bifidobacteria and reducing Enterobacteria may aid with colic symptoms. Vaginally born infants also suffer with colic, and these results showed the exhibited higher levels of Enterobacteria. B. breve M-16V® may be able to reduce the risk of colic in both vaginally and C section babies.
Use of probiotics in NICU’s is increasing. In some centres, probiotic use has seen NEC and infection rates drop significantly. Although premature birth complications are complex, it is postulated that many of these conditions are in some way related to the microbiome. As premature infants have immature guts with typically low levels of Bifidobacteria and high levels of inflammation this could be plausible.
A potential theory to explain how B. breve M-16V® may decrease NEC incidence comes from the reduction of a short chain fatty acid (SCFA) know as butyrate. SCFA’s are produced by bacteria via the fermentation of undigested carbohydrates and proteins in the gut. They are generally associated with health and well-being but in preterm infants, overproduction may not be beneficial. Butyrate is produced from specific microbes in the gut such as Clostridia, Bacteroides and Escherichia spp. Butyrate may contribute to NEC in many different ways, from immune responses, inflammation and increased mucosal injury (Wang C et al., 2007).
Additionally, by increasing levels of Bifidobacteria through B. breve M-16V® supplementation, this will help lower levels of butyrate via competition with butyrate producers alongside modulating inflammatory markers.
During the early years, the microbiome helps to train the immune system, allowing it to develop and distinguish between friend and foe. Atopic dermatitis (AD) and asthma are heavily associated with one another and are often present in infancy. It’s been suggested that 40% of children with AD develop asthma in later childhood (Gustafsson D et al., 2000). Studies have shown that microbial imbalances in the gut may be associated with the onset of allergies and atopy. Low levels of Bifidobacteria and Lactobacilli and high levels of Escherichia coli and Clostridium difficile have both been associated with the development of eczema or atopy (Kalliomaki M et al., 2001) (Abrahamsson TR et al., 2012) (Melli L et al., 2016).
Eczema rates in the UK are high. Majority of eczema cases are diagnosed by age 1 (Cork M J et al., 2019). Although the exact cause of eczema is unknown, there are likely to be many factors involved, including the microbiome. Increased gut permeability and imbalanced immune responses could contribute. The World Allergy Organisation (WAO) also took into consideration that probiotics for eczema may be beneficial (Fiocchi A et al., 2015).
Asthma is one of the most common health conditions, especially in children (Lin J et al., 2018). It is thought probiotics may support asthma due to immune regulation and modulation of inflammatory responses.
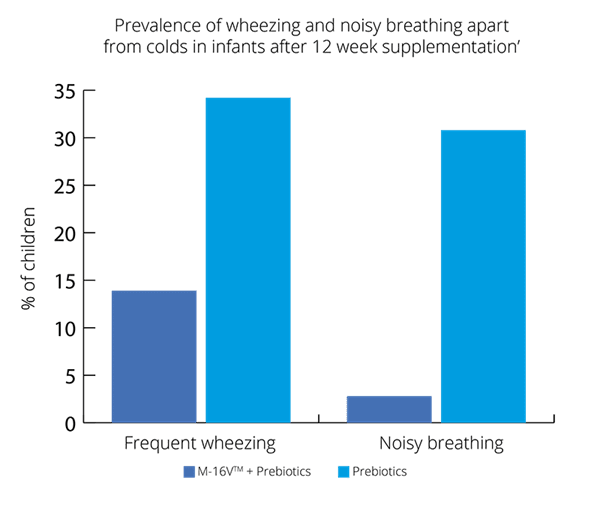
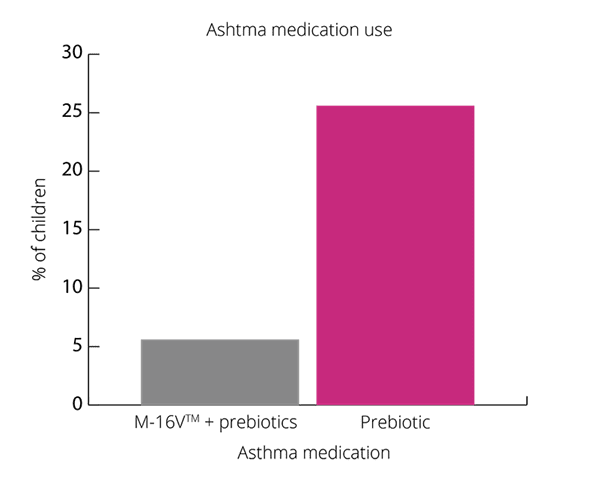
Overall, results show promising results for B. breve M-16V® in children with asthma like symptoms (Van der Aa et al., 2011).
The exact mechanism for how B. breve M-16V® improves asthma and eczema symptoms is unclear. It is thought that it may be due to the ability of the strain to modulate the immune system and improve inflammatory responses and Th1/Th2 ratio.
As well as promoting a healthy gut environment and microbiome. B. breve M-16V® may also support digestive symptoms.
B. breve M-16V® has been heavily researched for its probiotic benefits in infants. However, there are a few studies demonstrating its immune modulating effects in adults.
Both these studies show promise for B. breve M-16V® use in adults with AD or asthma. However further research with higher numbers of participants is needed to fully understand the benefits.
B. breve M-16V® is a highly researched strain that has been trialled in thousands of infants. What makes this strain so unique is the extensive research in premature babies, highlighting its safety and efficacy. There is a clear interaction between B. breve M-16V® and the immune system, the strain has been shown to regulate inflammatory responses and help balance Th1/Th2 ratios, all helping to reduce atopy and allergies. The strongest clinical evidence lies in the strains ability to boost Bifidobacteria levels. It is probable that the strain is able to compete with pathogens residing in the gut and create an environment more favourable to indigenous beneficial bacterial strains. It would be of interest to conduct more randomised, controlled trials on the strain and to fully understand the mechanisms of action at play. All in all, the strain has demonstrated its ability to improve gut health, the microbiome and immune system in infants.
Authors: Information on this strain was gathered by Dr Kate Stephens PhD Food and Microbial Sciences; Gut Microbiology (University of Reading), BSc Medical Microbiology; and Kerry Beeson, BSc (Nut.Med) Nutritional Therapist.
Last updated: 25th May 2020
As some properties & benefits of probiotics may be strain-specific, this database provides even more detailed information at strain level. Read more about the strains that we have included from this genus below.
Bifidobacterium lactis strains: Bifidobacterium lactis Bi-07®, Bifidobacterium lactis BB-12®, Bifidobacterium lactis HN019 and Bifidobacterium lactis Bl-04®.
Bifidobacterium infantis strains: Bifidobacterium infantis 35624.
Bifidobacterium breve strains: Bifidobacterium breve M-16V®.
For more information and the latest research on probiotics,, please visit the Probiotic Professionals pages.
For products containing this strain visit the Optibac Probiotics shop.
Abrahamse-Berkeveld M et al. (2016). Infant formula containing galacto and fructo oligosaccharides and Bifidobacterium breve M-16V supports adequate growth and tolerance in healthy infants in a randomised, controlled, double blind and prospective multi centre study. Journal Of Nutritional Science, 5: 1-13.
Abrahamsson TR et al. (2012). Low diversity of the gut microbiota in infants with atopic eczema. J. Allergy Clin. Immunol, 129, 434-440.
Akiyama K et al. (1994). Effects of oral administration of Bifidobacteria breve on development of intestinal microflora in extremely premature infants. Acta Neonatol. Jpn, 30: 130-137.
Athalye-Jape G et al. (2017). Bifidobacterium breve M-16V as a probiotic for pretern infants: A strain specific review. Journal of Parenteral and Enteral Nutrition, 42 (4): 677-688.
Chua MC et al. (2017). Effect of synbiotic on the gut microbiota of Cesarean delivered infants. A randomised, double blind, multicenter study. Journal of Pediatric Gastroenterology and Nutrition, 65 (1): 102-106.
De Weerth C et al. (2013). Intestinal microbiota of infants with colic: development and specific signatures. Pediatrics, 131 (2): 550-558.
Del Guidice MM et al. (2017). Bifidobacterium mixture (B. longum BB536, B infantis M-63 and B. breve M-16V) treatment in children with seasonal allergic rhinitis and intermittent asthma . Ital. J. Pediatri, 43, 25.
Dubois N et al. (2016). Characterising the intestinal microbiome in infantile colic: findings based on an integrative review of the literature . Biological Research for Nursing , 18 (3): doi:10.1177/1099800415620840.
Enomoto T et al. (2014). Effects of bifidobacterial supplementation to pregnant women and infants in the prevention of allergy development in infants and on faecal microbiota. Allergol Int, 63 (4): 575-85.
Ezaki et al. (2012). Prophylactic probiotics reduce cows milk protein intolerance in neonates after small intestine surgery and antibiotic treatment presenting symptoms that mimics postoperative infection. Allergology International, 61 (1): 107-113.
Fujii T et al. (2006). Bifidobacterium breve enhances transformation growth factor B1 signalling by regulating smad7 expression in preterm infants. J. Pediatri. Gastroenterol. Nutr, 43: 83-88.
Giannetti E et al. (2017). A mixture of 3 Bifidobacteria decreases abdominal pain and improves the quality of life in children with irritable bowel syndrome. A multi center, randomised, double blind, placebo controlled, crossover trial. Journal of Clinical Gastroenterology, 51 (1): 5-10.
Gustafsson D et al. (2000). Development of allergies and asthma in infants and young children with atopic dermatitis- a prospective follow up to 7 years of age. . Allergy, 55: 240-245.
Hattori K et al. (2003). Effects of administration of bifidobacteria on faecal microflora and clinical symptoms in infants with atopic dermatitis . Arerugi, 5, 387.
Hikaru U et al. (2010). Bifidobacteria prevents preterm infants from developing infection and sepsis. International Journal of Probiotics and Prebiotics, 5 (1) 33-36.
Hikaru U et al. (2010). Bifidobacteria prevents preterm infants from developing infection and sepsis. International Journal of Probiotics and Prebiotics , 5 (1): 33-36.
Hulshof L et al. (2018). Exploring immune development in infants with moderate to severe atopic dermatitis. frontiers in Immunology, doi: 10.3389/immu.2018. 00630.
Ishizeki S et al. (2013). Effect of administration of bifidobacteria on intestinal microbiome in low birth weight infants and transition of administered bifidobacteria: a comparison between one species and three species administration . Anaerobe , 23: 38-44.
Kalliomaki M et al. (2001). Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J. Allergy Clin. Immunol, 107, 129-134 .
Kosuwon P et al. (2018). A synbiotic mixture of scGOS/lcFOS and Bifidobacterium breve M-16V increases faecal Bifidobacterium in healthy young children. Beneficial Microbes, 541-552.
Lee SA et al. (2015). Comparison of the gut microbiota profile in breast fed and formula fed korean infants using pyrosequencing . Nutr Res Pract, 9 (3): 242-248.
Li Y et al. (2004). Effects of Bifidobacterium breve supplementation on intestinal flora of low birth weight infants. Pediatr. Int, 46: 509-515.
Makino H. (2018). Bifidobacterial strains in the intestines of newborns originate from their mothers. Bioscience of Microbiota, Food and Health, 37 (4): 79-85.
Melli L et al. (2016). Intestinal microbiota and allergic diseases: a systermatic review. Allergol. Immunopathol, 44, 177-188.
Patole et al. (2014). Effect of Bifidobacterium breve M-16V supplementation on faecal Bifidobacteria in preterm neonates- a randomised double blind placebo controlled trial . PLoS one, 9 (3): e89511.
Patole et al. (2016). Benefits of Bifidobacterium breve M-16V supplementation in preterm neonates- a retrospective cohort study. PLoS ONE, 11: E0150775.
Sakata H et al. (1985). Development of the intestinal flora in very low birth weight infants compared to normal full-term newborns. Eur J Pediatri, 144: 186-90.
Satoh Y et al. (2007). Bifidobacteria prevents nectrotising enterocolitis and infection in preterm infants. . International Journal of Probiotics & Prebiotics, 2; 49.
Tamburini S et al. (2016). The microbiome in early life: implications for health outcomes. Nature Medicine , 22 (7): 713-722.
Taniuchi S et al. (2005). Administration of Bifidobacterium to infants with atopic dermatitis: changes in faecal microflora and clinical symptoms. The Journal of Applied Research, 5 (2): 387-396.
Thavagnanam S et al. (2008). A meta-analysis of the association between Caesrean section and childhood asthma . Clinical and experimental allergy, 38 (4): 629-633.
Turroni et al. (2012). Diversity of bifidobacteria within the infant gut microbiota. PLoS ONE, 7: e36957.
Turroni F et al. (2009). Exploring the diversity of the bifidobacterial population in the human intestinal tract . Appl. Environ. Microbiol, 75: 1534-1545.
Van de Pol M et al. (2010). Synbiotics reduce allergen-induced T helper 2 response and improve peak expiratory flow in allergic asthmatics . Allergy, 66 (1): 39-47.
Van der Aa et al. (2011). Synbiotics prevent asthma-like symptoms in infants with atopic dermatitis. Allergy , 170-177.
Wang C et al. (2007). Effects of oral administration of Bifidobacterium breve on faecal lactic acid and short chain fatty acids in low birth weight infants. Journal of Pediatric gastroenterology and nutrition, 44: 252-257.
Wong B et al. (2019). Exploring the Science behind Bifidobacterium breve M-16V in infant health. Nutrients, 11 (8); 1724 doi: 10.3390/nu11081724.
Yoshida Y et al. (2010). Clinical effects of probiotic Bifidobacterium breve supplementation in adult patients with atopic dermatitis. Yonago Acta medica , 53: 37-45.