When this strain was originally isolated, it was thought to be a member of the species Bifidobacterium bifidum. More modern classification techniques reclassified it as Bifidobacterium animalis and then finally to Bifidobacterium lactis. In fact, it was decided that this strain did not fulfil the criteria for a species but rather a subspecies; strictly speaking making it Bifidobacterium animalis subsp. lactis; yet which is now more commonly referred to as Bifidobacterium lactis BB-12®.
The weight of research which supports the use of this strain suggests that it may have numerous potential benefits for health, and it is of interest to note that one of the largest clinical trials ever conducted on a probiotic strain was performed using Bifidobacterium lactis BB-12®. Also of interest is the fact that several of the trials mentioned here were performed using this strain in combination with Lactobacillus acidophilus LA-05.
Bifidobacterium lactis BB-12® is a bacterial strain found in food supplements. B. lactis BB-12® has been shown to reach the gut alive through confirmed microbial changes in the stools and faecal recovery. In one particular study, by Uchida, K. et al (2015), B. lactis BB-12® was identified in stool samples in 57% of participants, for the duration of B. lactis BB-12® supplementation.
The same study ran an ‘excessive ingestion safety test’, which demonstrated no difference in incidences of GI complaints between when participants were and were not taking the supplement in this arm of the trial, which led to the conclusion that the reported symptoms could not have been induced by B. lactis BB-12® intake.
Additional safety studies, such as those performed by Saavedra, J. (2004) and Vlieger, AM. et al (2009) have looked at B. lactis BB-12® as a food supplement in young infants.
In a gold standard trial by Saavedra et al (2004) 118 infants aged 3-24 months took either B. lactis BB-12® and S. thermophilus supplemented formula or placebo for 18 months. Investigating long term intake of these strains, they assessed infant growth, general wellbeing and intestinal health as well as tolerance and adverse events. Overall, the B. lactis BB-12® and S. thermophilus supplement was well tolerated, showing no significant differences compared to placebo with regards to growth rate, health care attention or day-care absenteeism. Additionally, the B. lactis BB-12® and S. thermophilus supplemented group saw improvements in infant wellbeing, with reduced rates of colic and irritability reported.

Bifidobacterium lactis BB-12® is a well-established, highly researched strain of probiotic, believed to be one of the most clinically researched strains available commercially. This strain is one of the most respected probiotics featured in this database and, as previously mentioned, is probably best known for being used in one of the largest ever clinical trials conducted on a probiotic strain. This particular trial assessed the effect of BB-12® on improving bowel regularity in constipated participants, where it was found to significantly alleviate constipation.
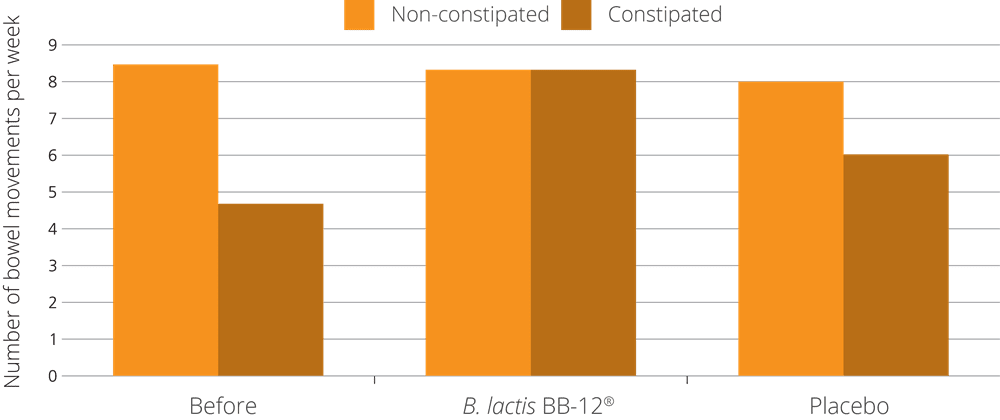
The B. lactis BB-12® strain was put to the test in research that used over 1,200 volunteering participants from all over Europe, including France, the UK and Germany. This fact in itself is interesting, as it meant that the trial encompassed a variety of different diets and lifestyles. The subjects were all healthy and had never been diagnosed with any acute digestive function; however, they had all reported symptoms of low defecation frequency and abdominal discomfort.
The volunteers were split into 3 groups: the first group received a 1 billion dose of B. lactis BB-12® once daily, the second group received 10 billion dose of B. lactis BB-12® once daily, and the third group received a placebo. The groups were monitored over a period of 4 weeks. Results showed that the 1 billion B. lactis BB-12® group revealed the most statistically impressive result, improving defecation frequency from 3 days a week to 4.5 days a week (Eskesen et al. 2015).
Once again it is confirmed that the BB-12 probiotic strain can bring significant gastrointestinal benefits to those who experience digestive issues.' Mikkel Jungerson, Scientific Advisor, HHN, Chr. Hansen
The aim of another clinical trial was to compare the effectiveness of B. lactis BB-12® to three other strains of friendly bacteria, for women suffering with constipation, and interestingly also in women not suffering with constipation, to ensure B. lactis BB-12® did not result in diarrhoea in those who are not constipated. 35 women aged between 20-39 years old were divided into two groups: those who were constipated and those who were non-constipated. Half of the women in each group were given B. lactis BB-12® plus the other three probiotic strains, and the other half were given just the three probiotic strains without the B. lactis BB-12®. In those who were given the three strains plus the B. lactis BB-12®, for those who had constipation, B. lactis BB-12® was able to harmonise regularity of bowel movements and significantly increase stool frequency, and in the non-constipated women it did not show an increase in bowel regularity (Nishida et al, 2004). This trial demonstrates that B. lactis BB-12® is superior in helping with constipation compared to the other three strains used in this study, and that it should not cause diarrhoea, unlike many common laxatives. (Nishida et al, 2004)
A triple-blind randomized controlled trial was conducted on 60 constipated pregnant women who were randomly put into two groups. One group received 300g of yoghurt with 48 billion units of BB-12® and L. acidophilus LA-05® probiotic strains added, the other group received a placebo yoghurt. The participants were monitored for symptoms of constipation. The frequency of defecation was increased from 2.1 (0.8) at the start of the study to 8.3 (4.4) in the probiotic yoghurt group vs. 2.3 (0.7) at to 8.1 (4.3) in the placebo yoghurt group at the end of the 4th week. The researchers concluded that a probiotic yoghurt containing these two strains could therefore play a role in improving the symptoms of constipation in pregnancy (Mirghafourvand M et al, 2016).
Further relevant studies: Kabeerdoss et al. (2011), Kajander et al. 2008), Malpeli et al (2012), Matsumoto et al. (2001), Murakami et al. (2006), Murakami et al. (2006), Pitkala, et al. (2007), Simrén et al. (2010), Søndergaard et al. (2011), Uchida et al. (2005).
A randomised, double-blind, placebo-controlled trial was conducted involving 209 elderly nursing home residents. The participants were randomised into three groups. The first received 1 billion CFU/day of Bifidobacterium longum strains, the second 1 billion CFU/day of B. lactis BB-12® and a third group received a placebo, all in a fermented oat drink, for 7 months. Regularity and consistency of bowel moments were recorded. Bowel movements were significantly improved and normalized in both probiotic groups compared to the placebo group (Pitkälä KH et al., 2007). In another study, a combination of Lactobacillus acidophilus LA-05® and Bifidobacterium lactis BB-12® was shown to help alleviate chronic constipation in elderly patients. The participants either received unfermented milk as a placebo or fermented milk containing the probiotic strains. The group who received the probiotic combination experienced a significant improvement in frequency of bowel movements compared to the baseline group (Alm L, 1993).
It’s believed that probiotic bacteria in the gut may interact with the immune system in several different ways: they may increase local and systemic antibody production; increase immune cell activity, and modulate signals in epithelial and immune cells. Research into the effect of B. lactis BB-12® on immune function has suggested that it may be able to help reduce respiratory infections.
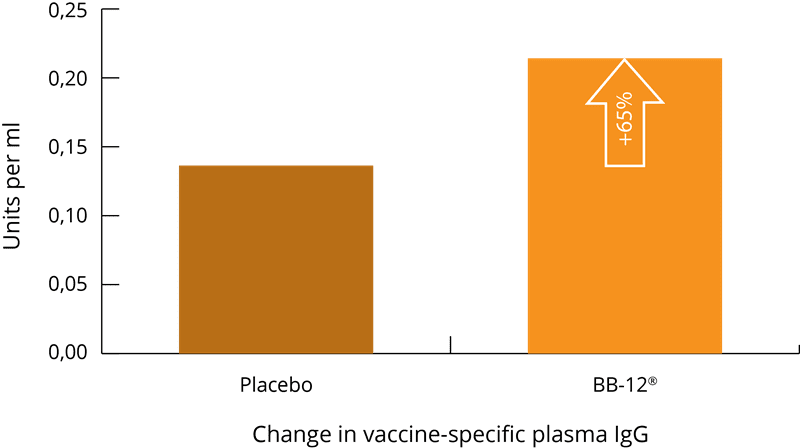
A randomised, double-blind, placebo-controlled, parallel-group study was conducted using 211 participants who consumed either a B. lactis BB-12® (capsule), a dairy drink containing L. paracasei CASEI 431®, or a matching placebo once daily for 6 weeks. After 2 weeks, a seasonal influenza vaccination was given. Plasma and saliva samples were collected at baseline and after 6 weeks in order to analyse antibodies, cytokines and innate immune parameters. Changes in the vaccine-specific plasma IgG, IgG1 and IgG3 were significantly greater in both probiotic groups versus the placebo group. This suggests that supplementation with B. lactis BB-12® or L. paracasei CASEI 431® may be an effective means to improve immune function (Rizzardini, 2012).
Further relevant studies: Holsher et al (2012), Hoppu et al (2012), Kabeerdoss (2011), Kloster et al (2008), Latvala (2008), Lópeza et al (2010), Merenstein et al (2010), Nova et al (2011), Ouwehand et al (2008), Rautava et al (2009), Rizzardini (2012), Schiffrin et al (1997), Smith T. et al (2012), Taipale et al (2011), Valsecchi et al (2014), Weizman et al (2005).
B. lactis BB-12® has also been tested in pregnant women to assess its potential to prevent or reduce atopic sensitisation in their offspring.
In a randomized, double-blind trial a total of 415 pregnant women were first given either probiotic milk or placebo from 36 weeks of pregnancy to 3 months postnatally during breastfeeding. The probiotic milk contained Lactobacillus rhamnosus GG® , Lactobacillus acidophilus LA-05® and Bifidobacterium animalis subsp. lactis BB-12®. The results showed a measurable reduction in the cumulative incidence of atopic dermatitis in their children (Dotterud et al, 2010).
B. lactis BB-12® was again trialled in pregnant women in another study by Laitinen et al (2009) which focused on the potential of dietary changes alongside probiotics to positively affect the body’s management of blood sugar. Throughout the study period, dietary and probiotics interventions yielded consistently improved glucose metabolism and insulin sensitivity in 256 healthy, pregnant women. These results provided clinical evidence of a significant role for the gut microbiota in the healthy management of blood sugar both during and after pregnancy.
Further relevant studies: Asemi et al (2013), Mirghafourvand & Mojgan et al (2016)
A double-blind, placebo-controlled study looked at the efficacy of a milk formula supplemented with B. lactis BB-12® for the prevention of diarrhoea in infants. This study, which involved 90 infants younger than eight months suggested that B. lactis BB-12® may statistically lower the chances of infants developing diarrhoea (Chouraqui et al 2004).
Further relevant studies: Chouraqui et al (2004), Hol et al. (2008), Kowalska-Duplaga et al (2004), Larsen (2006), Nopchinda S et al (2002), Saavedra et al (1994).
In one study, 160 patients were either given triple therapy to eradicate Helicobacter pylori or triple therapy with a combined probiotic including B. lactis BB-12®. The study concluded that supplementing with a probiotic yoghurt can improve the intention-to-treat eradication rates of H. pylori, and can restore the depletion of Bifidobacteria in stool samples after triple therapy (de Vrese et al, 2011).
Further relevant study: Sheu et al (2006), de Vrese et al (2011), Wang et al (2004).
A study to assess the effects of probiotics on antibiotic associated diarrhoea (read more about probiotics with antibiotics on the Probiotics Learning Lab) was conducted using 88 patients who were infected with H. pylori but who were otherwise healthy. The subjects were all given antibiotic therapy for eradication of the H. pylori, but a group of these were also given a milk containing B. lactis BB-12® and L. acidophilus LA-05® . It was found that the probiotic group had much decreased intestinal symptoms and the duration of their antibiotic associated diarrhoea was reduced (Sheu et al, 2002).
These results were supported by a further study conducted by Bhalla (2011) which used a probiotic formula containing the strains B. lactis BB-12® and L. acidophilus LA-05® . The findings indicated that these strains of probiotic could significantly reduce the severity of antibiotic-associated diarrhoea.
Further relevant studies: Fox et al (2015), Wenus et al (2008).
In one study, 27 exclusively breast-fed infants with atopic eczema were split into three groups. One group was weaned to a probiotic-supplemented whey formula containing B. lactis BB-12®; the second group was weaned to the same formula but containing Lactobacillus rhamnosus GG® (ATCC 53103), and the third group was a control group weaned to the same formula without probiotics. The result was a significant improvement in skin condition in those children who had been given the probiotic as opposed to those who had not (Solauri et al, 2000).
Further relevant study: Dotterud et al., (2010).
Oxidative stress is known to be a factor in the pathophysiology of diabetes, and research has indicated that probiotics may have a role in reducing oxidative stress. This was demonstrated by Ejtahed et al (2012) in a trial using 64 subjects with type 2 diabetes mellitus. For six weeks, one group received probiotic yoghurt containing Lactobacillus acidophilus LA-05® and Bifidobacterium lactis BB-12®, and a control group received conventional yoghurt. The probiotic group showed significantly lower fasting blood glucose, highlighting a role for probiotics in the management of diabetes.
Impaired lipid metabolism in diabetics has been the focus of further studies. Another trial by Ejtahed et al (2011) monitored 60 male and female subjects with type 2 diabetes and elevated cholesterol (LDL-C) for six weeks. The control group was given 300g of conventional yoghurt daily, and an intervention group was given 300g of probiotic yoghurt containing Lactobacillus acidophilus LA-05® and Bifidobacterium lactis BB-12®. The probiotic group showed a 4.54% decrease in total cholesterol and a 7.45% decrease in LDL-C compared with control, suggesting that probiotics may improve cardiovascular disease risk factors in type 2 diabetics.
Further relevant studies: Asemi et al (2013), Ejtahed et al (2011), Laitinen et al (2009), Mohamadshahi et al, (2014), Sadrzadeh-Yeganeh et al (2011).
For products containing this strain visit the Optibac Probiotics shop.
Authors: Information on this strain was gathered by Joanna Scott-Lutyens BA (hons), DipION, Nutritional Therapist; and Kerry Beeson, BSc (Nut.Med) Nutritional Therapist.
Last updated - 25th May 2020
As some properties & benefits of probiotics may be strain-specific, this database provides even more detailed information at strain level. Read more about the strains that we have included from this genus below.
Bifidobacterium lactis strains: Bifidobacterium lactis Bi-07®, Bifidobacterium lactis HN019 and Bifidobacterium lactis Bl-04®.
Bifidobacterium infantis strains: Bifidobacterium infantis 35624.
Bifidobacterium breve strains: Bifidobacterium breve M-16V®.
Read more about the research behind Lactobacillus rhamnosus GG® and Lactobacillus acidophilus LA-05® .
For more information and the latest research on probiotics, please visit the Probiotic Professionals pages.
Alandera M, (2001), ‘Effect of galacto-oligosaccharide supplementation on human faecal microflora and on survival and persistence of Bifidobacterium lactis BB-12® in the gastrointestinal tract’, International Dairy Journal, 11(10):817–825.
Alm L, et al (1993) Effect of a new fermented milk product "CULTURA" on constipation in geriatric patients. 1st Lactic Acid Bacteria Computer Conference Proceedings. Horizon Scientific Press, Norfolk, England 1993.
Asemi Z. et al (2013), ‘Effect of daily consumption of probiotic yoghurt on insulin resistance in pregnant women: a randomized controlled trial’, Eur J Clin Nutr. Jan. 67(1):71-4.
Bhalla et al (2011), ‘A randomized, double blind, placebo controlled multi-centric trial on efficacy of Providac techsules (Lactobacillus acidophilus LA-5 and Bifidobacterium lactis BB-12®) for the prevention of antibiotic associated diarrhoea in Indian patients, American College of Clinical Pharmacy (ACCP): Chicago Illinois.
Chouraqui J.P. et al (2004), ‘Acidified milk formula supplemented with Bifidobacterium lactis: impact on infant diarrhoea in residential care settings’, J Pediatr Gastroenterol Nutr., Mar. 38(3):288-92.
Dotterud C.K. et al (2010), ‘Probiotics in pregnant women to prevent allergic disease: a randomized, double-blind trial’, Br J Dermatol., Sep. 163(3):616-23.
Ejtahed H.S. et al (2012), ‘Probiotic yogurt improves antioxidant status in Type 2 diabetic patients’, Nutrition. May: 28(5):539-43.
Ejtahed H.S. et al (2011), ‘Effect of probiotic yogurt containing Lactobacillus acidophilus and Bifidobacterium lactis on lipid profile in individuals with type 2 diabetes mellitus’, J Dairy Sci. 94(7):3288-94.
Eskesen, D. et al. (2015), ‘Effect of the probiotic strain Bifidobacterium animalis subsp. lactis BB-12® on defecation frequency in healthy subjects with low defecation frequency and abnormal discomfort: a randomised, double-blind, placebo-controlled, parallel-group trial’, British Journal of Nutrition, Nov 28: 114(10) :1638–1646.
Fox MJ, Ahuja KDK, Robertson IK, et al, (2015), ‘Can probiotic yogurt prevent diarrhoea in children on antibiotics? A double-blind, randomised, placebo controlled study’, BMJ Open 2015; 5:e006474. Doi: 10.1136/bmjopen-2014- 006474.
Hol J. et al., (2008), ‘The acquisition of tolerance toward cow's milk through probiotic supplementation: a randomized, controlled trial’, J Allergy Clin Immunol, 121(6):1448-54.
Holscher et al (2012), ‘Effects of Prebiotic-Containing Infant Formula on Gastrointestinal Tolerance and Fecal Microbiota in a Randomized Controlled Trial’,JPEN J. Parenter Enteral NutrJanuary 201236:95S-105S.
Holscher H. et al (2012), ‘Bifidobacterium lactis BB-12® Enhances Intestinal Antibody Response in Formula-Fed Infants’, Journal of Parenteral and Enteral Nutrition, 36:1_suppl: 106S - 117S.
Hoppu U. et al (2012), ‘Probiotics and dietary counselling targeting maternal dietary fat intake modifies breast milk fatty acids and cytokines’, Eur J Nutr, Mar; 51(2) :211-9.
Isolauri E. (2000), ‘Probiotics in the management of atopic eczema’, Clin Exp Allergy. Nov 30 (11):1604-10.
Kabeerdoss (2011), ‘Effect of yoghurt containing Bifidobacterium lactis BB-12® on faecal excretion of secretory immunoglobulin A and human beta-defensin 2 in healthy adult volunteers’,. Nutr J. Dec 23:10:138.
Kajander K. et al., (2008), ‘Clinical trial: multispecies probiotic supplementation alleviates the symptoms of irritable bowel syndrome and stabilizes intestinal microbiota’, Aliment Pharmacol Ther, 21(1):48-57
Kloster H. et al., (2008), ‘Effect of a probiotic milk product on gastrointestinal and respiratory infections in children attending day-care’, Microbial Ecology in Health and Disease, 20: 80-85.
Kowalska-Duplaga, K. et al., (2004), ‘Efficacy of Trilac® in the treatment of acute diarrhoea in infants and young children-a multicentre, randomized, double-blind placebo-controlled study’, Pediatria Wspolczesna, 6(3):295-300.
Laitinen K .et al (2009), ‘Probiotics and dietary counselling contribute to glucose regulation during and after pregnancy: a randomised controlled trial’, Br J Nutr. Jun: 101(11):1679-87.
Larsen C.N. et al., (2006), ‘Dose-response study of probiotic bacteria Bifidobacterium animalis subsp lactis BB-12 and Lactobacillus paracasei subsp paracasei CRL-341 in healthy young adults’, Eur J Clin Nutr., 60(11):1284-93.
Latvala, S., et al (2008), ‘Potentially probiotic bacteria induce efficient maturation but differential cytokine production in human monocyte-derived dendritic cells’, World Journal of Gastroenterology, Sep 28; 14(36):5570–5583.
Lópeza P. et al (2010), ‘Distinct Bifidobacterium strains drive different immune responses in vitro’, International Journal of Food Microbiology 31 March: 138 (1–2):157–165.
Malinen E. et al (2002), ‘PCR- ELISA II: Analysis of Bifidobacteria populations in human faecal samples from a consumption trial with Bifidobacterium lactis BB-12® and a galacto-oligosaccharide preparation’, Syst Appl Microbiol., Aug; 25(2):249-58.
Malpeli A. et al (2012), ‘Randomised, double-blind and placebo-controlled study of the effect of a synbiotic dairy product on orocecal transit time in healthy adult women’, Nutr Hosp. Jul-Aug: 27(4):1314-9.
Matsumoto, M. et al. (2001), ‘Effect of Yoghurt with Bifidobacterium lactis BB-12® in Improving Fecal Microflora and Defecation of Healthy Volunteers’, Journal of Intestinal Microbiology, 14(2):97-102.
Merenstein D.J. et al (2010) ‘The study to investigate the potential benefits of probiotics in yogurt, a patient-oriented, double-blind, cluster-randomised, placebo-controlled, clinical trial’, Eur J Clin Nutr., Jul: 64(7):685-91.
Merenstein D.J. et al (2015), ‘Safety of Bifidobacterium animalis subsp. lactis (B. lactis) strain BB-12® supplemented yogurt in healthy adults on antibiotics: a phase I safety study’, Gut Microbes, 6(1):66-77.
Mirghafourvand Mojgan et al (2016), “The Effect of Probiotic Yogurt on Constipation in Pregnant Women: A Randomized Controlled Clinical Trial.” Iranian Red Crescent Medical Journal, Nov; 18(11): e39870 PMC. Web.
Mohamadshahi et al, (2014), ‘Effects of probiotic yogurt consumption on lipid profile in type 2 diabetic patients: A randomized controlled clinical trial’. Journal of Research in Medical Sciences : The Official Journal of Isfahan University of Medical Sciences, 19(6):531-536.
Murakami, T. et al (2006), ‘Safety and effect of yoghurt containing Bifidobacterium lactis BB-12® on improvement of defecation and faecal microflora in healthy volunteers’, Journal of Nutritional Food, 9:15-26.
Nishida, S. et al. (2004), ‘Effect of Yoghurt Containing Bifidobacterium lactis BB-12® on Improvement of Defecation and Fecal Microflora of Healthy Female Adults’, Milk Science, 53(2):71-80
Nopchinda S et al (2002), ‘Effect of Bifidobacterium lactis BB12® with or without Streptococcus thermophilus supplemented formula on nutritional status,’ J Med Assoc Thai. Nov. 85 (Suppl 4):S1225-31.
Nova E. et al., (2011), ‘Beneficial effects of a synbiotic supplement on self-perceived gastrointestinal well-being and immunoinflammatory status of healthy adults’, J Med Food, 14(1-2):79-85.
Ouwehand A.C. et al., (2008), ‘Bifidobacterium microbiota and parameters of immune function in elderly subjects’, FEMS Immunol Med Microbiol, 53(1):18-25.
Pitkala, K.H et al. (2007), ‘Fermented cereal with specific Bifidobacteria normalizes bowel movements in elderly nursing home residents. A randomized, controlled trial’, Journal of Nutritional Health and Aging, 11(4):305-311.
Rautava S. et al (2009), ‘Specific probiotics in reducing the risk of acute infections in infancy-- a randomised, double-blind, placebo-controlled study’, Br J Nutr. June, 101(11):1722-6.
Rautava S. et al (2012), ‘Probiotics modulate host-microbe interaction in the placenta and fetal gut: a randomized, double-blind, placebo-controlled trial’, Neonatology, 102(3):178-84.
Rizzardini G. (2012), ‘Evaluation of the immune benefits of two probiotic strains Bifidobacterium animalis ssp. lactis BB-12® and Lactobacillus paracasei ssp. paracasei, Lactobacillus casei 431® in an influenza vaccination model: a randomised, double-blind, placebo-controlled study’, British Journal of Nutrition, March, 107(6):876-884.
Saavedra J.M. et al (1994), ‘Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhoea and shedding of rotavirus’, The Lancet, October, 344(8929):1046-9.
Saavedra et al. 2004. Long-term consumption of infant formulas containing live probiotic bacteria: tolerance and safety. The American journal of clinical nutrition. 9:261–7.
Sadrzadeh-Yeganeh H. et al., (2010), ‘The effects of probiotic and conventional yoghurt on lipid profile in women’, Br J Nutr., 103(12):1778-83.
Satokari R.M. et al (2001), ‘Polymerase chain reaction and denaturing gradient gel electrophoresis monitoring of fecal bifidobacterium populations in a prebiotic and probiotic feeding trial’, Syst Appl Microbiol, 24(2):227-31.
Schiffrin et al. (1997), ‘Immune modulation of blood leukocytes in humans by lactic acid bacteria: criteria for strain selection’, Am J Clin Nutr.,66(2):515S-520S.
Sheu, B.-S., et al (2002), ‘Impact of supplement with Lactobacilli and Bifidobacteria- containing yogurt on triple therapy for Helicobacter pylori eradication’, Alimentary Pharmacology & Therapeutics, 16 pp. 1669–1675.
Sheu B.S. et al (2006), ‘Pretreatment with Lactobacilli and Bifidobacterium-containing yogurt can improve the efficacy of quadruple therapy in eradicating residual Helicobacter pylori infection after failed triple therapy’, Am J Clin Nutr. April, 83(4):864-9.
Simrén M. et al., (2010), ‘Clinical trial: the effects of a fermented milk containing three probiotic bacteria in patients with irritable bowel syndrome - a randomized, double-blind, controlled study’, Aliment Pharmacol Ther., 31(2):218-27.
Smith T, (2012), ‘Effect of Lactobacillus rhamnosus LGG® and Bifidobacterium animalis ssp. lactis BB-12® on health-related quality of life in college students affected by upper respiratory infections’, British Journal of Nutrition, 109:1999-2007.
Søndergaard B. et al., (2011), ‘Effects of probiotic fermented milk on symptoms and intestinal flora in patients with irritable bowel syndrome: a randomized, placebo-controlled trial’, Scand J Gastroenterol, 46(6):663-72.
Taipale T. et al, (2011), ‘Bifidobacterium animalis subsp. lactis BB-12® in reducing the risk of infections in infancy’, British Journal of Nutrition, 105:409-416.
Taipale T. et al (2007), ‘Dissolution of xylitol from a food supplement administered with a novel slow-release pacifier: preliminary results’, Eur Arch Paediatr Dent., June 8 (2):123-5.
Taipale T. et al (2012), ‘Bifidobacterium animalis subsp. lactis BB-12® administration in early childhood: a randomized clinical trial of effects on oral colonization by Streptococcus mutans and the probiotic’, Caries Res. 46(1):69-77.
Uchida et al. (2005), ‘Effect of fermented milk containing Bifidobacterium lactis BB-12® on stool frequency, defecation, fecal microbiota and safety of excessive ingestion in healthy female students’, Journal of Nutritional Food, 8(1):39-51.
Valsecchi C. et al (2014), ‘Evaluation of the effects of a probiotic supplementation with respect to placebo on intestinal microflora and secretory IgA production, during antibiotic therapy, in children affected by recurrent airway infections and skin symptoms’, J Biol Regul Homeost Agents, Jan-Mar, 28(1):117-24.
Vlieger et al. 2009 Tolerance and safety of Lactobacillus paracasei ssp. paracasei in combination with Bifidobacterium animalis ssp. lactis in a prebiotic-containing infant formula: a randomised controlled trial.
Wang K.Y. et al., (2004), ‘Effects of ingesting Lactobacillus- and Bifidobacterium-containing yogurt in subjects with colonized Helicobacter pylori’, Am J Clin Nutr., 80(3):737-41.
de Vrese M, et al (2011), ‘Probiotic Lactobacilli and Bifidobacteria in a fermented milk product with added fruit preparation reduce antibiotic associated diarrhoea and Helicobacter pylori activity, Journal of Dairy Research, 78:396-403.
Weizman Z. et al., (2005), ‘Effect of a probiotic infant formula on infections in child care centers: comparison of two probiotic agents’, Pediatrics, 115(1):5-9.
Wenus C. et al (2008), ‘Prevention of antibiotic-associated diarrhoea by a fermented probiotic milk drink’, Eur J Clin Nutr., February, 62(2):299-301.
Zakharova I.N., Berezhnaya I.V., Skorobogatova E.V. Fermented milk-based baby drinks fortified by prebiotics and probiotics: impact on the health of infants and young children. Russian Journal of Woman and Child Health. 2022;5(3):253–261 (in Russ.).