

The Protectis® strain is a member of the Lactobacillus reuteri species; it is also known as Lactobacillus reuteri DSM 17938. This strain is the daughter strain of Lactobacillus reuteri ATCC 55730, a bacteria which was originally isolated from the breast milk of a Peruvian mother. As of April 2020 L. reuteri has been officially reclassified to Limosilactobacillus reuteri and so the full strain is now also known as Limosilactobacillus reuteri Protectis® (Zheng J et al 2020).
The L. reuteri species of Lactobacilli has been regularly featured in research into the use of probiotics for the support of digestive health, but it is of particular interest for use in paediatric digestive disorders such as infantile colic (Urbańska & Szajewska 2014). Additionally, it has been researched for oral health, usually in combination with the strain Lactobacillus reuteri ATCC PTA 5289.
Lactobacillus reuteri DSM 17938 is often administered to children as a food supplement. As such, safety has been determined in children as well as adults. In one study this strain and another strain (Bifidobacterium lactis BB-12®) were administered separately to infants by adding to their milk formula. Both strains were well tolerated with no side effects reported, and consistent results in growth, feeding, stool characteristics and behaviour were observed compared to the control group which received plain formula milk with no supplementation (Weizman, Z. & Alsheikh, A., 2006). In adults L. reuteri DSM 17938 has been administered in high doses of 100 billion CFU and found to be safe, as judged by lack of gastrointestinal symptoms, normal blood results for haematology and chemistries, normal urinalysis and physical examination (Wolf, B. W. et al., 1995).
The ability of this strain to survive to reach the gut alive has also been investigated, again in children. A trial by Abrahamsson, T. et al., in 2009 found a high level of colonisation of L. reuteri in stool samples of infants taking Lactobacillus reuteri DSM 17938 supplementation in their first year of life. The authors are confident to assume this increase reflects survival of the L. reuteri DSM 17938 strain through the gut.

Due to the nature of its source (from human breast milk) this probiotic has been extensively researched for use in paediatric digestive support, and is probably best known for its potential benefits in this area. A randomised, controlled study attempted to determine the efficacy of L. reuteri Protectis® in reducing infantile colic, and thereby also reducing parental concerns due to the associated symptoms. The authors studied 105 infants aged 10 days or less, who were randomly assigned to receive either five drops of a supplement containing the probiotic with 400IU of vitamin D, or five drops of vitamin D only, daily for a period of three months. The results illustrated that those children who were given the supplement containing the probiotic L. reuteri Protectis® were 75% and 96% less likely to use two different pain relieving agents. Overall, there were also 40% fewer phone calls to paediatricians, and 63% less use of infant formula than in the control group. The authors therefore concluded L. reuteri Protectis® reduced parental distress and was effective in the prevention of colic in healthy infants (Savino F. et al., 2015).
In another randomised, double-blind, placebo-controlled trial, the focus was to look at the efficacy of Lactobacillus reuteri Protectis® for the treatment of infantile colic, specifically in breast-fed infants. This trial was conducted in Canada where 52 infants with colic were randomly chosen to receive either the probiotic L. reuteri Protectis®, or a placebo, for a period of 21 days. Daily periods of crying and ‘unsettled’ times were recorded. It was found that, in the probiotic group, periods of colic-related unsettled behaviour were significantly shorter, and there was a significant reduction in daily crying times at the end of treatment period, compared with infants in the placebo group. On day 21, a significantly higher proportion of infants in the L. reuteri Protectis® group responded to treatment with a ≥ 50% crying time reduction compared with infants given placebo (Chau K. et al., 2014).
Other Related Studies: Ashraf M.W. et al., (2015), Calderon V.V. et al., (2014), Indrio F. et al., (2014), Mi G.L. et al., (2015), Savino F. et al., (2007), Savino F. et al., (2010), Sung V. et al., (2014), Szajewska H. et al., (2013), Roos S. et al., (2013).
Abdominal pain is a distressing symptom for many children, and its causes can be difficult to determine. It is believed that probiotics may help to alleviate and prevent this type of digestive issue, and Lactobacillus reuteri Protectis® is one of the strains that has been tested for this purpose.
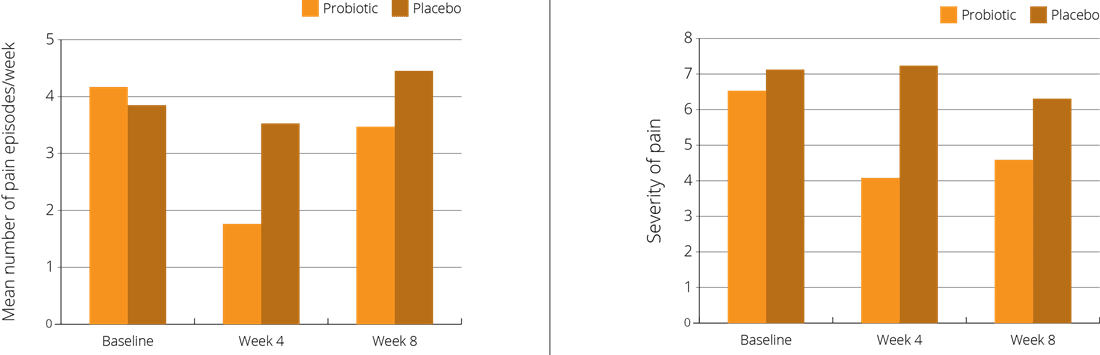
A double-blind, placebo-controlled trial focused on 101 children, aged 6-15 years, who had been diagnosed as suffering from Functional Abdominal Pain (FAP). The children were randomly assigned to receive either a supplement containing the probiotic L. reuteri Protectis® at a dose of 100 million CFUs once daily, or a placebo. The intervention period was four weeks, with a follow up at eight weeks. Children in the intervention group reported significant relief in both the frequency and severity of abdominal pain and bloating symptoms, compared to those in the placebo group (Weizman Z. et al., 2016).
Acute infectious diarrhoea in children is a potentially serious condition often requiring hospitalisation, as prolonged episodes can result in severe dehydration. Scientists are exploring a role for probiotics in the support of this condition, to try to reduce the incidence and duration of symptoms.
With this focus, a multi-centre, randomised, single-blind trial used 64 children aged between 3 months and 5 years who were being treated in their homes for acute diarrhoea. All of the children received conventional rehydration therapy, but roughly half of the infants were also randomised to receive a supplement containing Lactobacillus reuteri Protectis® for five days. Compared to the control group, who received the rehydration therapy only, the duration of diarrhoea was significantly reduced, and at 48 hours after supplementation, 55% of the children were free from diarrhoea compared to just 13% in the control group (Dinleyici E.C. et al., 2015).
A further randomised, double-blind, placebo-controlled study was conducted to evaluate whether daily administration of Lactobacillus reuteri Protectis® could reduce the frequency and duration of episodes of diarrhoea and other health outcomes in children. A total of 336 healthy children, who were aged between 6 months and 3 years and all attending nurseries, were randomised to receive either a supplement containing Lactobacillus reuteri Protectis® each day, or a placebo, for a period of three months. A follow up was then conducted after a three month period without supplementation. It was found that the use of the L. reuteri Protectis® probiotic significantly reduced the number of episodes, and the duration, of diarrhoea symptoms compared to baseline. It was also shown to reduce the incidence of respiratory tract infections in healthy children (Gutierrez-Castrellon P. et al., 2014).
Other Related Studies: Ceratto S. et al., (2014), Coccorullo P. et al., (2010), Dinleyici E.C., et al., (2014), Dinleyici E.C. et al., (2015), Eftekhari K. et al., (2015), Francavilla R. et al., (2012), Indrio F. et al.m (2011), Olgaç M.A.B. et al., (2013), Olgaç M.A.B. et al., (2013), Romano C. et al., (2014), Urbanska M., (2015), Wanke M., (2012).
The Protectis® (DSM 17938) strain has also been trialled in adults, and has been found to offer some benefits for bowel regularity. Constipation indiscriminately affects individuals of all ages and gender, and can have a profound impact on their quality of life.
A double-blind, placebo controlled trial was conducted using 40 adults, who had all been diagnosed with functional constipation. For the purposes of the trial, the patients were randomly selected to receive a supplement containing Lactobacillus reuteri Protectis®, or a placebo, for 4 weeks.
There was no significant change in stool consistency between the control and treatment groups; however, a statistically significant increase in the number of weekly bowel movements was noted. The frequency of bowel movements per week in the probiotic increased from 2.68 at the start of the study to 5.28 at the end of the four week study period. The mean increase in bowel movements at week 4 was 2.6 in the probiotic group compared to 1.0 in the placebo group (Ojetti V., 2014).
Other Relevant Studies: Coccorullo P., (2010), Olgaç M.A.B., (2013).
Helicobacter pylori is considered to be a pathogenic species. Though, in many people, it is benign and lies dormant in the stomach mucosal surface without causing symptoms, in some individuals it proliferates and can cause inflammation of the stomach lining. H. pylori infection is a common factor in the development of stomach ulcers, and once identified, the conventional treatment is a triple therapy drug combination, including two different types of antibiotics and a proton pump inhibitor. Due to the ability of this bacterial species to burrow deep into the stomach lining, it may escape the effects of the medication, and so the triple therapy is not always 100% effective. For this reason, other effective solutions are being explored, and the potential of probiotics to help increase the efficacy of conventional treatment is being considered. As a result, many of the top probiotic strains included in this database have been featured in studies to assess their efficacy against this problematic pathogen.
An open trial was conducted to try to determine if the Proton Pump Inhibitor (PPI) Pantoprazolein in combination with L. reuteri Protectis® (rather than antibiotics) would effectively resolve H. pylori infection in adults. A total of 22 subjects with Helicobacter pylori infection were given a dose of the probiotic alongside their proton pump inhibitor treatment. It was found that twice daily doses of the L. reuteri Protectis® probiotic plus the PPI medication successfully eradicated H. pylori infection in 13.6 % (3/22) of patients. The authors concluded that ‘L. reuteri [Protectis®] may have a potential role in H. pylori eradication therapy if the cure rate can be improved by changes in dose, dosing interval, or duration of therapy’ (Dore M.P. et al., 2014).
Other Relevant Studies: Emara M.H. et al., (2013), Francavilla R.F. et al., (2013), Kotzev I.A. et al., (2015), Lionetti E., (2006).
Although the majority of resident bacteria in humans are found in the intestines, populations of microflora are to be found throughout the body. This has led to a growing interest in the use of probiotics to help rebalance the microbiota in areas outside the digestive system, including the oral mucosa.
With this aim, a double blind, placebo-controlled study looked at 45 women with pregnancy gingivitis, who were otherwise healthy. The participants were examined to check the extent of the gingivitis and their plaque index, and they were then told to continue with their usual oral health routines. They were then randomly given lozenges to be consumed twice daily in their last trimester – the lozenges contained both L. reuteri ATCC PTA 5289 and L. reuteri Protectis®. The results showed that both gingivitis and the plague index were significantly reduced in the probiotic lozenge group compared to the placebo group (Schlagenhauf U. et al., 2016).
Another study looked at the effect of lozenges containing the two strains L. reuteri Protectis® and L. reuteri ATCC PTA 5289 on 215 elderly people with oral Candida overgrowth (thrush). In this double-blind, randomised, placebo-controlled trial, patients were given either two probiotic lozenges a day, or a placebo, for a period of 12 weeks. It was found that there was a statistically significant reduction (53%) in the prevalence of Candida counts in the lozenge group compared to the placebo group (Kraft-Bodi E. et al., 2015).
Other Related Studies: Cannon M. et al., (2013), Cildir S. et al., (2012), Flichy-Fernández A.J. et al., (2015), Gizani S. et al., (2015), Hallström H. et al., (2015), Ince G. et al., (2015), Iniesta M. et al., (2012), Keller M.K. & Twetman S., (2012a), Keller M.K. et al., (2012b), Keller M.K, et al., (2012c), Keller M.K.,. (2014), Szkaradkiewicz A.K. et al., (2014), Tekçe M. et al., (2015), Teughels W. et al., (2013), Vicario M. et al., (2012), Vivekananda M.R. et al.. (2010), Vestman N.R. et al., (2013), Vestman N.R. et al., (2015).
There has been an increasing level of interest in the use of probiotic bacteria to support immune function. In a randomised, double-blind, placebo controlled trial, the effect of L. reuteri Protectis® on airway inflammation in 43 asthmatic children was assessed.
The children were aged between 6 and 14 years, and had well-controlled asthma symptoms. They were randomly selected to receive a supplement containing L. reuteri Protectis® or a placebo, for a period of 60 days. There were 22 children in the active group and 21 in the placebo group, and the results were measured clinically and by certain inflammatory parameters. It was noted that, compared to the placebo group, the probiotic significantly reduced bronchial airway inflammation without change in clinical parameters (Miraglia et al., 2012).
Other Related Studies: Abrahamsson T.R. et al., (2009), Abrahamsson T.R. et al., (2011), Abrahamsson T.R. et al., (2013), Böttcher M.F. et al., (2008), Ciprandi G., (2015), Mangalat N. et al., (2012).
Authors: Information on this strain was gathered by Joanna Scott-Lutyens BA (hons), DipION, Nutritional Therapist; and Kerry Beeson, BSc (Nut.Med) Nutritional Therapist.
Last updated - 7th May, 2020
As some properties & benefits of probiotics may be strain-specific, this database provides even more detailed information at strain level. Read more about the strains that we have included from this genus below.
Lactobacillus acidophilus strains: Lactobacillus acidophilus LA-05, Lactobacillus acidophilus NCFM®, Lactobacillus acidophilus Rosell-52.
Lactobacillus casei strains: Lactobacillus casei Shirota, Lactobacillus casei DN-114001.
Lactobacillus plantarum strains: Lactobacillus plantarum LP299v.
Lactobacillus reuteri strains: Lactobacillus reuteri RC-14®.
Lactobacillus rhamnosus strains: Lactobacillus rhamnosus LGG®, Lactobacillus rhamnosus HN001, Lactobacillus rhamnosus GR-1® and Lactobacillus rhamnosus Rosell-11.
Lactobacillus paracasei strains: Lactobacillus paracasei CASEI 431®.
For more info and the latest research on probiotics, please visit the Probiotic Professionals pages.
For products containing these strains visit the Optibac Probiotics shop.
Ashraf M.W. et al., (2015), ‘Probiotics are effective in alleviating infantile colic; results of a randomized controlled trial held at Benazir Bhutto Hospital, Rawalpindi, Pakistan’. Rawal Medical Journal, 40(3):277-280.
Abrahamsson T.R. et al., (2009), ‘Probiotic lactobacilli in breast milk and infant stool in relation to oral intake during the first year of life’. J. Pediatr. Gastroenterol Nutr., 49: 349-354.
Abrahamsson T.R. et al., (2011), ‘A Th1/Th2-associated chemokine imbalance during infancy in children developing eczema, wheeze and sensitization’. Clin Exp Allergy, 41:1729-1739.
Abrahamsson T.R. et al., (2013), ‘No effect of probiotics on respiratory allergies: a seven-year follow up of a randomised controlled trial in infancy’. Pediatr Allergy Immunol. 24: 556–561. (Follow-up data of the Abrahamsson 2007 trial).
Böttcher M.F. et al., (2008), ‘Low breast milk TGF- β2 is induced by Lactobacillus reuteri supplementation and associates with reduced risk of sensitization during infancy’. Pediatr Allergy Immunol., 19:497-504. (Additional results of the Abrahamsson 2007 trial).
Cannon M. et al., (2013), ‘Effectiveness of CRT at measuring the salivary level of bacteria in caries prone children with probiotic therapy’. J. Clin. Pediatr Dent., 38(1):55-60.
Calderon V.V. et al., (2014), ‘Valuation the use of Lactobacillus reuteri in the treatment of infant colic: a pilot study’. Acta Pediatr Esp, 72:154-159.
Ceratto S. et al., (2014), ‘Atopic disorders, asthma, migraine and BMI Z-score in children treated with Lactobacillus reuteri for infantile colic: a post hoc analysis’. JPGN, 58 (S1):500-501.
Chau K. et al., (2014), ‘Probiotics for infantile colic: a randomized double-blind placebo-controlled trial investigating Lactobacillus reuteri DSM 17938’. J. Pediatr., 166:74-78.
Cildir S. et al., (2012), ‘A novel delivery system of probiotic drop and its effect on dental caries risk factors in cleft lip/palate children’ Cleft Palate Craniofac J. 49:369-372 (online 10 Feb. 2011).
Ciprandi G. & Varricchio A., (2015), ‘Vitamin D3 plus Lactobacillus reuteri DSM 17938 as adjuvant for allergen immunotherapy: a preliminary experience’. Jacobs Journal of Allergy and Immunology, 2(1):014.
Coccorullo P. et al., (2010), ‘Lactobacillus reuteri (DSM 17938) in infants with functional chronic constipation: a double-blind, randomized, placebo controlled study’. J Pediatrics, 157: 598-602.
Dumitru I.M. et al., (2009), ‘The effect of Lactobacillus reuteri therapy in intestinal disorders of various causes in HIV-infected patients’. Infectio ‘ro, 20(4):18- 21.
Dinleyici E.C., et al., (2014) ‘Lactobacillus reuteri DSM 17938 effectively reduces the duration of acute diarrhoea in hospitalised children’. Acta Paediatr. 103:e300-e305.
Dinleyici E.C. et al., (2015), ‘Lactobacillus reuteri DSM 17938 shortens acute infectious diarrhea in a pediatric outpatient setting’. J. Pediatr, 91(4):392-396.
Dore M.P. et al., (2014), ‘Lactobacillus reuteri in the treatment of Helicobacter pylori infection’. Intern. Emerg. Med., 9:649-654.
Emara M.H. et al., (2013), ‘Lactobacillus reuteri in management of Helicobacter pylori infection in dyspeptic patients: a double-blind placebo-controlled randomized clinical trial’. Therapeutic Advances in Gastroenterology, 7:4-13.
Flichy-Fernández A.J. et al., (2015), ‘The effect of orally administered probiotic Lactobacillus reuteri-containing tablets in peri-implant mucositis: a double-blind randomized controlled trial’ J Periodontal Res., 50(6):775-8.
Francavilla R. F. et al., (2013), ‘Lactobacillus reuteri strain combination in Helicobacter pylori infection: a randomized, double-blind, placebo-controlled study’. J. Clin. Gastroenterol., 48:407-413.
Eftekhari K. et al., (2015), ‘A randomized double-blind placebo-controlled trial of Lactobacillus reuteri for chronic functional abdominal pain in children’. Iran J. Pediatr., 25(6):e2616.
Francavilla R. et al., (2012), ‘Randomised clinical trial: Lactobacillus reuteri DSM 17938 vs. placebo in children with acute diarrhoea - a double-blind study’. Aliment Pharmacol Ther., 36: 363–369.
Georgieva M. et al., (2015), ‘Use of the probiotic Lactobacillus reuteri DSM 17938 in the prevention of antibiotic-associated infections in hospitalized Bulgarian children: a randomized, controlled trial’. J. of IMAB, 21(4):895-900.
Gizani S. et al., (2015), ‘Effect of the probiotic bacterium Lactobacillus reuteri on white spot lesion development in orthodontic patients’. Eur J. Orthod., 38(1):85-89.
Gutierrez-Castrellon P. et al., (2014), ‘Diarrhea in preschool children and Lactobacillus reuteri: a randomized controlled trial’. Pediatrics, 133:e904-e909.
Hallström H. et al., (2015), ‘Probiotic supplements and debridement of peri-implant mucositis: A randomized controlled trial’. Acta Odontol Scand., 74(1):60-6.
Ince G. et al., (2015), ‘Clinical and biochemical evaluation of Lactobacillus reuteri containing lozenges as an adjunct to non-surgical periodontal therapy in chronic periodontitis’ J Periodontol. 86:746-754 (Additional results of the Tekçe 2015 trial).
Indrio F. et al., (2014), ‘Prophylactic use of a probiotic in the prevention of colic, regurgitation, and functional constipation. A randomised clinical trial’. JAMA Pediatr., 168:228-233.
Indrio Fet al. (2011) ‘Lactobacillus reuteri accelerates gastric emptying and improves regurgitation in infants’ Eur J Clin Invest. 41:417-422. (L. reuteri DSM 17938)
Iniesta M. et al., (2012), ‘Probiotic effects of orally administered Lactobacillus reuteri-containing tablets on the subgingival and salivary microbiota in patients with gingivitis. A randomized clinical trial’ J Clin Periodontol., 39: 736–744.
Keller M.K. & Twetman S., (2012a), ‘Acid production in dental plaque after exposure to probiotic bacteria’ BMC Oral Health, 12(1):44.
Keller M.K. et al., (2012b), ‘Effect of chewing gums containing the probiotic bacterium Lactobacillus reuteri on oral malodour’ Acta Odont Scand., 70:246-50.
Keller M.K. et al., (2012c), ‘Probiotic supplements (Lactobacillus reuteri DSM 17938 and ATCC PTA 5289) do not affect regrowth of Mutans streptococci after full-mouth disinfection with chlorhexidine: a randomized controlled multicenter trial’. Caries Res. 46:140-146.
Keller M.K. et al., (2014), ‘Effect of tablets containing probiotic bacteria (Lactobacillus reuteri) on early caries lesions in adolescents: a pilot study’. Benef Microbes., 5(4):403-407.
Kotzev I.A. et al., (2015), ‘The effect of L. reuteri (ProGastria) on the eradication rate in elderly patients infected with H. pylori: a randomized, double-blinded, placebo controlled trial’. J. Prob. Health, 3:130.
Kraft-Bodi E. et al., (2015), ‘Effect of probiotic bacteria on oral Candida in frail elderly’. J Dent Res. 94(9):181S-186S.
Lionetti E., (2006), ‘Lactobacillus reuteri therapy to reduce side-effects during anti-Helicobacter pylori treatment in children: a randomised placebo controlled trial’. Aliment Pharmacol Ther., 24:1461-1468.
Mangalat N. et al., (2012), ‘Safety and tolerability of Lactobacillus reuteri DSM 17938 and effects on biomarkers in healthy adults: results from a randomized masked trial’. PLoS ONE, 9:e43910.
Mi G.L. et al., (2015) ‘Effectiveness of Lactobacillus reuteri in infantile colic and colicky induced maternal depression: a prospective single blind randomized trial’. Antonie Van Leeuwenhoek. 107:1547-1553.
Miraglia del Giudice M. et al., (2012), ‘Airways allergic inflammation and L. reuterii treatments in asthmatic children’. J. Biol Regul. Homeost Agents, 26(1):35(S)-40(S).
Ojetti V., (2014), ‘Effect of Lactobacillus reuteri supplementation in adults with chronic functional constipation: a randomised, double-blind, placebo-controlled trial’. J Gastrointestin Liver Dis.
23(4): 387-391.
Olgaç M.A.B. et al., (2013), ‘Comparison of probiotic and lactulose treatments in children
with functional constipation and determination of the effects of constipation treatment on quality of life.’ Turkish Pediatric Journal, 56: 1-7.
Olgaç M.A.B. et al., (2013), Lactobacillus reuteri DSM 17938 effectively reduces the duration of acute diarrhoea in hospitalised children’. Acta Paediatr. 103:e300-e305.
Romano C. et al, (2014,) ‘Lactobacillus reuteri in children with functional abdominal pain (FAP)’.
J. Paediatr Child Health, 50(10): E68-E71.
Roos S. et al., (2013), ‘454 pyrosequencing analysis on faecal samples from a randomized DBPC trial of colicky infants treated with Lactobacillus reuteri DSM 17938’. PLoS ONE, 8(2):e56710.
Savino F. et al. (2007) Lactobacillus reuteri ATCC 55730 versus Simethicone in the treatment of infantile colic: a prospective randomized study. Pediatrics 119:124-130.
Savino F, et al., (2010), ‘Lactobacillus reuteri DSM 17 938 in infantile colic: A randomized, double-blind, placebo-controlled trial’. Pediatrics 126: e526-e533.
Savino F. et al., (2015), ‘Preventive effects of oral probiotic on infantile colic: a prospective, randomised, blinded, controlled trial using Lactobacillus reuteri DSM 17938’. Benef. Microbes, 6(3):245-251, ePub 8 Dec. 2014.
Schlagenhauf U. et al., (2016) ‘Regular consumption of Lactobacillus reuteri-containing lozenges reduces pregnancy gingivitis: an RCT’. J Clin Periodontol. 43(11):948-954.
Schröder C. et al., (2015). ‘Effects of the regular intake of the probiotic Lactobacillus reuteri (DSM 17938) on respiratory and gastrointestinal infections in a workplace setting: a double-blind randomized placebo-controlled trial’. BMC Nutrition, 1:3.
Sung V. et al., (2014), ‘Treating infant colic with the probiotic Lactobacillus reuteri: double blind, placebo controlled randomised trial’. Brit. Med. Journal, 348:g2107.
Szajewska H. et al., (2013), ‘Lactobacillus reuteri DSM 17938 for the management of
infantile colic in breastfed infants: A randomized, double-blind, placebo-controlled trial’. J. Pediatr. 162:257-262.
Szkaradkiewicz A.K. et al., (2014), ‘Effect of oral administration involving a probiotic strain of Lactobacillus reuteri on pro-inflammatory cytokine response in patients with chronic periodontitis’. Arch Immunol Ther Exp., 62:495-500. Online 9 Feb. 2014.
Tekçe M. et al., (2015), ‘Clinical and microbiological effects of probiotic lozenges in the treatment of chronic periodontitis: a 1-year follow-up study’. J Clin Periodontol. 42:363-372, online ahead of print 3 March 2015, DOI: 10.1111/jcpe.12387.
Teughels W. et al., (2013), ‘Clinical and microbiological effects of Lactobacillus reuteri probiotics in the treatment of chronic periodontitis: a randomized placebo-controlled study’. J. Clin. Periodontol., 40:1025–1035.
Urbańska M. & Hania Szajewska H., (2014), ‘The efficacy of Lactobacillus reuteri DSM 17938 in infants and children: a review of the current evidence’. Eur J Pediatr., 173(10):1327–1337.
Urbanska M. (2015), ‘Effectiveness of Lactobacillus reuteri DSM 17938 for the prevention of nosocomial diarrhea in children: a randomized, double-blind, placebo-controlled trial’. Pediatr Infect Dis J., 35(2):142-5.
Vestman N. et al., (2013), ‘Lactobacillus reuteri influences regrowth of mutans streptococci after full-mouth disinfection: a doubleblind, randomised controlled trial’. Caries Res. 47:338-345. (Additional results of the Keller 2012 trial in Caries Res.).
Vestman N.R. et al., (2015), ‘Oral microbiota shift after 12-week supplementation with Lactobacillus reuteri DSM 17938 and PTA 5289; a randomized control trial’. PLoS ONE 10(5): e0125812. Published 6 May 2015, doi:10.1371/journal.pone.0125812.
Vicario M. et al., (2012) Clinical changes in periodontal subjects with the probiotic Lactobacillus reuteri Prodentis: A preliminary randomized clinical trial. Acta Odont Scand., 71(3-4):813-9.
Vivekananda M.R. et al., (2010), ‘Effect of the probiotic Lactobacilli reuteri (Prodentis) in the management of periodontal disease: a preliminary randomized clinical study’. J Oral Microbiol.,
2: 5344, DOI: 10.3402/jom.v2i0.5344, published 2 Nov.
Wanke M. & Szajewska H., (2012), ‘Lack of an effect of Lactobacillus reuteri DSM 17938 in preventing
nosocomial diarrhea in children: A randomized, double-blind, placebo-controlled trial’. J Pediatr. 161:40- 43.e.
Weizman Z. et al., (2016), ‘Lactobacillus reuteri DSM 17938 for the management of functional abdominal pain in childhood: a randomized, double-blind, placebo-controlled trial’. J Pediatr. 174:160-164.
Weizman, Z. & Alsheikh, A., (2006) ‘Safety and tolerance of a probiotic formula in early infancy comparing two probiotic agents: a pilot study’. J Am Coll Nutr, 25(5):415-9.
Wolf, B. W. et al., (1995) ‘Safety and Tolerance of Lactobacillus reuteri in Healthy Adult Male Subjects’. Microbial Ecology in Health and Disease, 8(2): 41-50. DOI: 10.3109/08910609509141381.
Zheng J, Wittouck S. et al., (2020) 'A taxonmonic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae'. Int.J.Syst.Evol.Microbiol, 70(4): 2782-2858. DOI: 10.1099/ijsem.0.004107