

Microbiology profile:
Researched conditions:
Researched in: pregnant women, newborns, infants, children, adults, elderly
Keywords: Probiotic, pregnant women, overall support
Health during pregnancy is vital. Many pregnant women take supplements to support their babies’ health but may not realise that their own health can impact that of their baby’s both before and after birth. Research is highlighting the first thousand days of life to be essential in microbiome and immune system development. These first thousand days include the 270 days of pregnancy. Emerging research is showing probiotic supplementation from the first trimester onwards may have beneficial effects for both mother and baby.
The microbiome undergoes many changes throughout pregnancy. This is unsurprising due to the hormonal, immunological and metabolic changes that are happening. In the first trimester, both gut and vaginal microbiomes are relatively stable. From trimester 1 to trimester 3, more pronounced changes are seen.
There is a lot of research on the vaginal microbiome and pregnancy. Microbial imbalances in the vaginal tract have been associated with pre-term births and spontaneous abortions (Peelan MJ et al., 2019). Increased microbial diversity and vaginal infections, such as with Gardnerella spp or Candida albicans, have been associated with pregnancy complications (Selma- Royo M et al., 2019). It is thought they may lead to an increase in pro-inflammatory cytokines and prostaglandins. This may stimulate uterine contractions and/or weaken foetal membranes. Fortunately studies have shown that the vaginal microbiome should become less diverse and lactobacilli dominant as the pregnancy progresses (Tamburini S et al., 2016) (Mesa M et al., 2020). This could be due to the rise in oestrogen. High oestrogen levels are associated with higher levels of glycogen in the vaginal tract, a major food source for lactobacilli. Lactobacilli can offer many protective effects against infections including the production of lactic acid, antimicrobials, and bio surfactants. These will all help to create an environment that is less favourable to pathogenic microbes thus inhibiting their growth. One study found that pregnant women in the last trimester had significantly different vaginal microbiomes to non pregnant women. Pregnant woman had higher levels of lactobacilli including Lactobacillus vaginalis, Lactobacillus crispatus and Lactobacillus jensenii. Whilst non pregnant woman exhibited a more diverse microbiome (Romero R et al., 2014).
There is little research on the gut microbiome changes throughout pregnancy, most studies focus on the vaginal, placental, and oral microbiomes. However, a study in 2012 found that pregnant women in trimester three exhibit gut microbiomes similar to that of individuals with diabetes or metabolic syndrome (Koren O et al., 2012). In the study, the scientists transferred stool samples from women in their first and third trimester into germ free mice. Stool from the first trimester resulted in little changes to the mouse. However, stools from the third trimester saw the mice gain weight, have insulin desensitisation and a different microbiome to that from trimester one. Further microbiome analysis of trimester one and three, showed as pregnancy progressed the microbiome reduced in diversity. The microbiome from trimester one resembled that of a healthy non pregnant woman. By trimester three, there were increases in inflammatory bacteria such as Proteobacteria, decreases in butyrate producers and increases in bacteria that give higher energy yields. Since this study, few studies have been conducted to confirm these results. If we do see these changes, we can conclude that this forms part of a normal pregnancy. The problems can arise if a woman is already predisposed to diabetes, in which case her risk of gestational diabetes is higher.
Microbiomes from the gut and vaginal microbiome likely transfer to the baby during labour. This explains why infants born from C sections and vaginal routes exhibit different microbiomes (Sheo Y et al., 2019). It is therefore important for expectant mothers to take care of their microbiomes during pregnancy. Diet, lifestyle, antibiotics, stress levels can all impact the microbiome which can result in dysbiosis and reduced passage of beneficial bacteria to the newborn.
Post labour, it’s been shown that vaginal and gut microbiomes may take time to return to ‘normal’ after birth. For the gut microbiome, it’s been estimated to take one month (Koren O et al., 2012). However, a study in the vaginal microbiome showed differences up to a year post birth (DiGiulio D et al., 2015) . However, instead of lactobacilli dominating, vaginal samples were abundant in many gut microbes suggesting migration during labour. This may increase the risk of intimate infections post pregnancy. There is also some evidence to suggest that post-natal depression may affect the quality of microbes present in breast milk which are passed to the new born (Browne P et al., 2019).
Therefore, health and well-being during pregnancy and postnatally is vital for women.
Human clinical trials on L. rhamnosus HN001 conducted in expectant mothers and during breastfeeding have shown no adverse effects on birth outcome; and no other adverse effects have been reported. This is highlighted throughout the assessment of trials in this review.
The gut-brain axis is an exploding research area connecting our mental health, wellbeing and behaviour to our microbial profile. Our microbiome may be able to interfere with neural pathways, neurotransmitter production and metabolism and central nervous systems. Postnatal depression is thought to affect approximately 10-15% of women (NHS, 2011). This number is predicted to be even higher as many woman often don’t realise they have it or do not report it. L. rhamnosus HN001 has been shown to reduce PND in mothers.
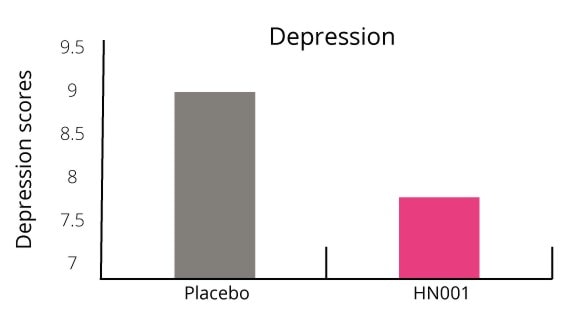
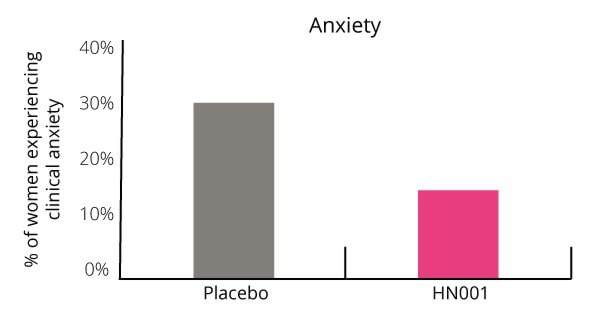
Additionally, the number of women who scored above the cut off point for clinical anxiety was 50% lower in the probiotic group (Graph 2). Only 15.6% of women suffered with clinical anxiety in the probiotic group compared with 29.4% in the placebo group.
This is one of the first studies to show an association between probiotic use and postnatal depression.
It is estimated that 8-24% of pregnant women may develop GDM (Farrar D et al., 2016). This risk can vary depending upon family history, previous GDM diagnosis, age, weight preconception and ethnicity. The microbiome may contribute to GDM due to the microbial changes outlined in the study by Koren et al., (Koren O et al., 2012). L. rhamnosus HN001 has been shown to reduce the risk of GDM, especially in pregnant women more at risk.
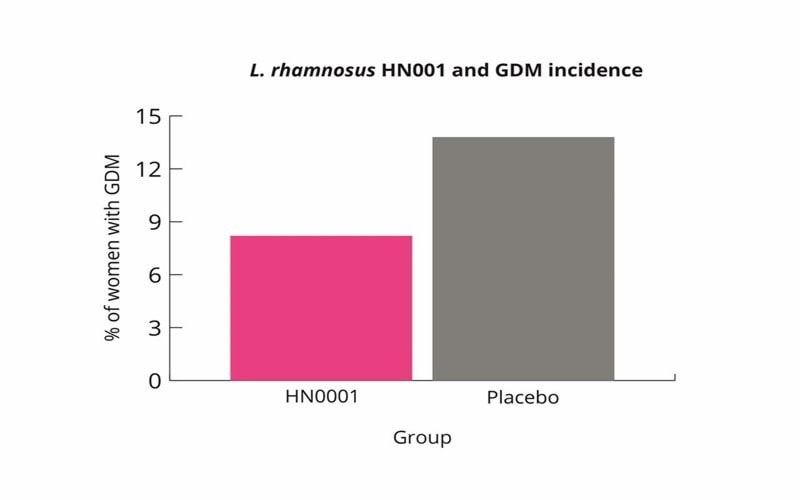
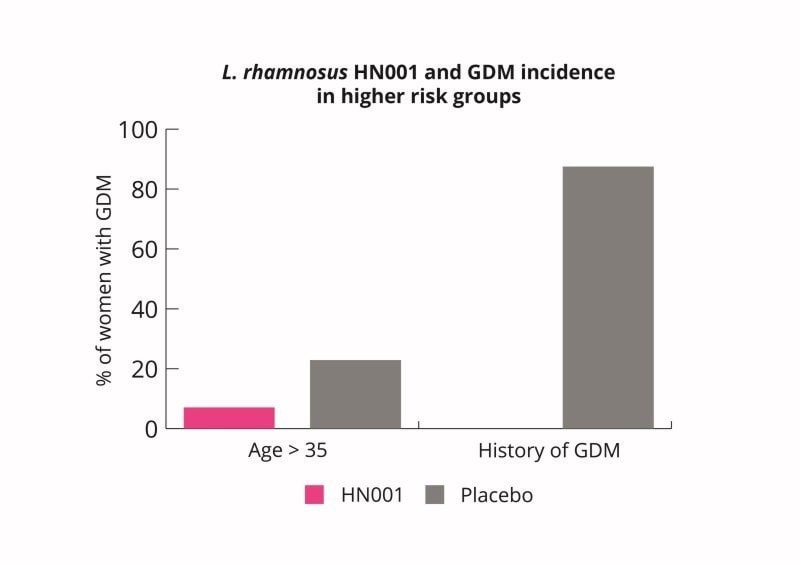
L. rhamnosus HN001 supplementation may therefore be a useful intervention for pregnant women to lower their risk of GDM. L. rhamnosus HN001 supplementation may be especially useful for woman at higher risk (Wickens K et al., 2017).
L. rhamnosus HN001 may enhance pregnant women’s immunity but it may also have some indirect benefits to foetal and/or newborn immune development.
Promoting good vaginal microbial health during pregnancy is important. Imbalances in the vaginal microbiome can lead to serious outcomes. Many pregnant women often suffer with intimate issues including bacterial vaginosis. This often requires a course of antibiotics which could impact the baby’s health. Antibiotic use in pregnancy has been shown to increase the risk of asthma, allergies and eczema in children (Risnes KR et al., 2011) (Hoskin-Parr L et al., 2013).
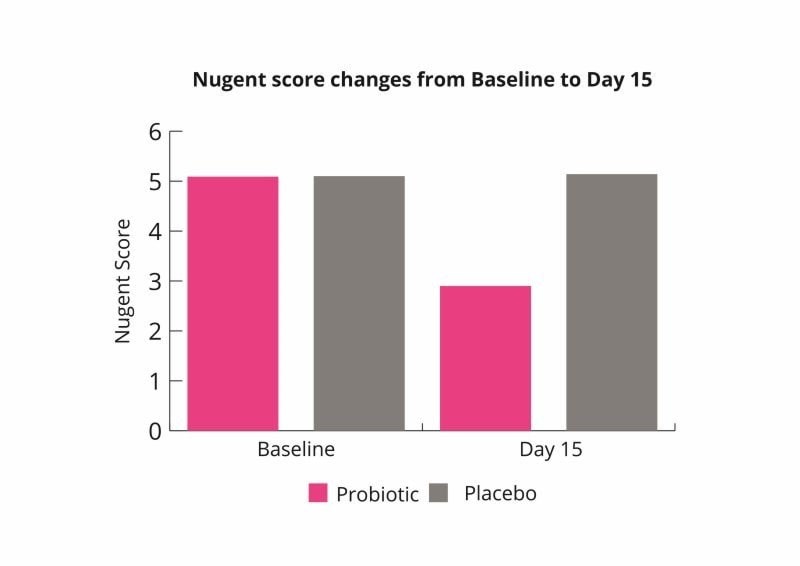
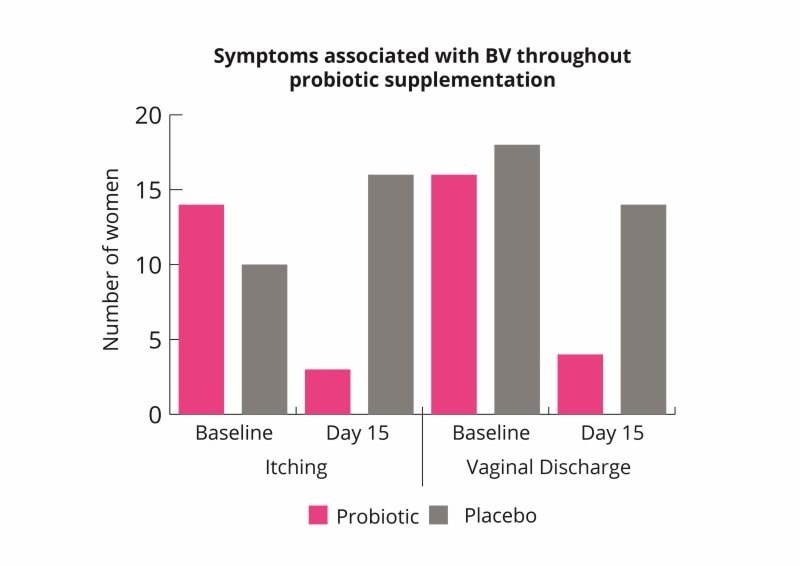
These results indicate that the probiotic combination may help to rebalance the vaginal microbiome and improve symptoms of bacterial vaginosis (Russo et al., 2019).
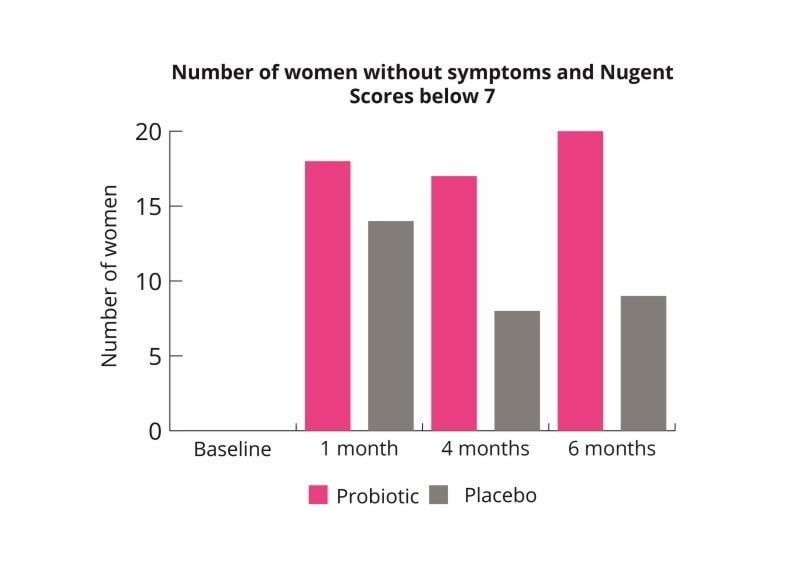
Eczema is very common in infants and has been associated with microbial imbalances in the gut and inflammation. Some studies have shown that probiotic supplementation to the mother followed by giving directly to the newborn may reduce the risk of eczema. Three studies followed up on one another (forming a six year study) found L. rhamnosus HN001 had significant benefits on the reduction of eczema.
Due to the promising results seen and protective effects of L. rhamnosus HN001, follow up studies were conducted.
Overall L. rhamnosus HN001 supplementation in the later part of pregnancy and direct supplementation to the infant from birth for two years was able to reduce the risk of eczema up to the age of six. This highlights the importance of the first few years of life. It provides a window of opportunity to intervene with probiotics to potentially reduce health conditions such as eczema and allergies.
Probiotic supplementation in pregnancy is an emerging area of research due to the importance of maternal health during gestation. Overall L. rhamnosus HN001 has been demonstrated to support many aspects of a woman’s health and wellbeing. It has been trialled extensively in pregnant women showing safety and efficacy. L. rhamnosus HN001 is one of the few strains to demonstrate abilities supporting mental wellbeing, vaginal health and immunity.
Authors: Information on this strain was gathered by Dr Kate Stephens PhD Food and Microbial Sciences; Gut Microbiology (University of Reading), BSc Medical Microbiology; and Kerry Beeson, BSc (Nut.Med) Nutritional Therapist.
Last updated: 15th May 2020
As some properties & benefits of probiotics may be strain-specific, this database provides even more detailed information at strain level. Read more about the strains that we have included from this genus below.
Lactobacillus acidophilus strains: Lactobacillus acidophilus LA-05, Lactobacillus acidophilus NCFM®, Lactobacillus acidophilus Rosell-52.
Lactobacillus casei strains: Lactobacillus casei Shirota, Lactobacillus casei DN-114001.
Lactobacillus plantarum strains: Lactobacillus plantarum LP299v.
Lactobacillus reuteri strains: Lactobacillus reuteri Protectis and Lactobacillus reuteri RC-14®.
Lactobacillus rhamnosus strains: Lactobacillus rhamnosus LGG®, Lactobacillus rhamnosus GR-1® and Lactobacillus rhamnosus Rosell-11.
Lactobacillus paracasei strains: Lactobacillus paracasei CASEI 431®.
For products containing this strain visit the Optibac Probiotics shop.
For more information and the latest research on probiotics, please visit the Probiotic Professionals pages.
Browne P et al. (2019). Human milk microbiome and maternal postnatal pyschosocial distress. Frontiers in Microbiology, 10, doi= 10. 3389/fmicb.2019.02333.
DeAlberti D et al. (2015). Lactobacilli aginal colonisation after oral consumption of Respecta complex: a randomised controlled pilot study. General Gyneacology , DOI: 10.1007/s00404-015-3711-4.
Dekker J et al. (2009). Safety aspects of probiotic bacterial strains Lactobacillus rhamnosus HN001 and Bifidobacterium animalis subs. lactis HN019 in human infants aged 0-2. International Dairy Journal, 149-154 .
Gill H et al. (2000). Enhancement of natural and acquired immunity by Lactobacillus rhamnosus (HN001), Lactobacillus acidophilus (HN017) and Bifidobacterium lactis (HN019). British Journal of Nutrition, 167-176.
Gill H et al. (2001). Immune enhancement conferred by oral delivery of Lactobacillus rhamnosus HN001 in different milk based substrates . Journal of Dairy Research , 68 (4): 611-616.
Koren O et al. (2012). Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell, 150 (3): 470-480.
Macaubas C et al. (2003). Association between antenatal cytokine production and the development of atopy and asthma at age 6 years. Lancet, 362: 1192-7.
Prescott SL et al. (2008). Supplementation with Lactobacillus rhamnosus or Bifidobacterium lactis probiotics in pregnancy increases cord blood IFNy and breast milk transforming growth factor B and immunoglobin A detection. Clinical and experimental Allergy , 38 (10): 1606- 1614.
Russo R et al. (2018). Study on the effects of an oral lactobacilli and lactoferrin complex in women with intermediate vaginal microbiota. Archives of Gynacology and Obstetrics, doi: 10.1007/s00404-0.18-4771-z.
Russo R et al. (2019). Randomised clinical trial in women with recurrent vulvovaginal candidiasis: efficacy of probiotics and lactoferrin as maintenance treatment. Mycoses, DOI: 10.1111/myc.12883.
Russo R et al. (2019b). Evidence based mixture containing Lactobacillus strains and lactoferrin to prevent recurrent bacterial vaginosis: a double blind, placebo controlled, randomised clinical trial. Beneficial Microbes, 10 (1): 19-26.
Selma- Royo M et al. (2019). Shaping Microbiota during the first 1000 days of Life. Information Security, DOI: 10.1007/5584_2018_312.
Sheih Y et al. (2001). Systemic immunity-enhancing effects in healthy subjects following dietary consumption of the lactic acid bacterium Lactobacillus rhamnosus HN001. Journal of the American College of Nutrition, 20 (2): 149-156.
Slykerman R et al. (2017). Effect of Lactobacillus rhamnosus HN001 in pregnancy on postpartum symptoms of depression and anxiety: a randomised double blind placebo controlled trial . EBioMedicine, 24, 159-165.
Tamburini S et al. (2016). The microbiome in early life: implications for health outcomes. Nature Medicine , 22 (7): 713-722.
Wickens K et al. (2008). A differential effect of 2 probiotics in the prevention of eczema and atopy: a double blind, randomised, placebo controlled trial. Atopic dermatitis and skin disease, 122 (4): 788-794.
Wickens K et al. (2013). A protective effect of lactobacillus rhamnosus HN001 against eczema in the first 2 years of life persists to age 4 years. Epidemiology of Allergic Disease , 42, 1071-1079.
Wickens K et al. (2013). Early supplementation with Lactobacillus rhamnosus HN001 reduces eczema prevalence to 6 years: does it also reduce atopic sensitisation? Epidemiology of Allergic Disease , 43: 1048-1057.
Wickens K et al. (2017). Early pregnancy probiotic supplementation with Lactobacillus rhamnosus HN001 may reduce the prevalence of gestational diabetes mellitus: a randomised controlled trial. British Journal of Nutrition, 117, 804-813.
Zheng J, Wittouck S. et al., (2020) 'A taxonmonic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae'. Int.J.Syst.Evol.Microbiol, 70(4): 2782-2858. DOI: 10.1099/ijsem.0.004107