

Lactobacillus acidophilus NCFM® is a very well-researched strain - certainly the most researched strain of the acidophilus species - which has been extensively tested in vitro, and also in both animal studies and in human clinical trials. There are actually over 45 clinical trials on this strain, a fact which confirms its position as one of the best probiotic strains in the world, with a well-deserved and a secure place in this database of the most well-researched probiotics on the planet. It has been used in a broad range of studies focusing on its potential to support many areas of gastrointestinal and general health, including immune function, antibiotic-associated gut disturbances, and the symptoms of Irritable Bowel Syndrome (IBS). The NCFM® strain is a member of the Lactobacillus acidophilus species.
The L. acidophilus NCFM® strain was first identified when isolated from a human source back in the early 1970's. Its strain name, NCFM®, is derived from an abbreviation of the “North Carolina Food Microbiology”, which is the name of the research laboratory at North Carolina State University (NCSU) where the strain was first discovered. You may also see Lactobacillus acidophilus NCFM® referred to as Lactobacillus acidophilus ATCC 700396, NCK56, N2 as well as NCFM®. This strain is often used in clinical trials in combination with Bifidobacterium lactis Bi-07®.
Lactobacillus acidophilus NCFM® is a food supplement with research demonstrating its safety and survival to reach the gut alive. In a study by Morovic, W. et al., (2017), L. acidophilus NCFM® alongside Bifidobacterium lactis Bl-04® and Bifidobacterium lactis Bi-07® was assessed for its safety in vivo. The results across a variety of parameters including antibiotic resistance, genomic risk factors, and acute toxicity confirmed all three strains to be safe for human consumption.
Lactobacillus acidophilus NCFM® has been isolated in stool samples after oral administration indicating survival through the gastro intestinal tract. This was recognised as early as 1978, as described in a paper by Gilliland, S. W. et al., (1978). More recently, in a study by Mai, V. et al., (2017) different types of capsule were used for administration, and regardless of the capsule type used L. acidophilus NCFM® was recovered in stool samples.
This strain has numerous studies to support its ability to improve overall wellbeing. Ringel-Kulka, T. et al., (2014); Ringel, Y. et al., (2008) and Lyra, A. et al., (2016).

Lactobacillus acidophilus NCFM® has been shown in multiple studies to help relieve the symptoms of pain and discomfort that are so typical in IBS sufferers. Clinical trials were able to measure a statistically significant improvement in symptoms, and also to determine the mechanism of action by which the improvement was achieved.
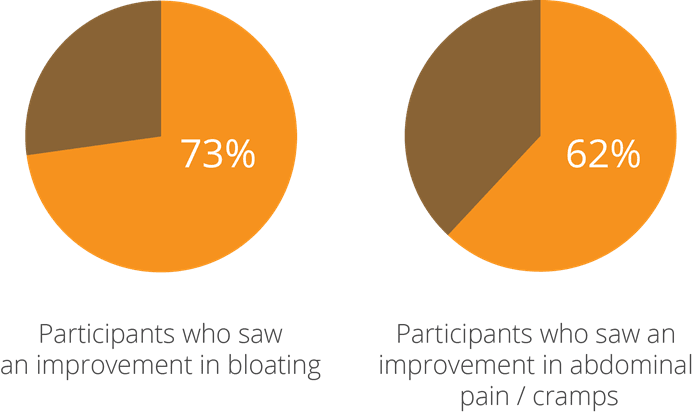
One of the earliest studies into this aspect of the strain’s potential, a double-blind, randomised, controlled clinical trial using 26 participants with IBS, yielded intriguing results and prompted further research to be done. At the beginning of the test period, subjects were given a questionnaire and a stool test, where low levels of beneficial bacteria and high levels of pathogens were noted. They were then given a supplement containing a combination of bacteria including Lactobacillus acidophilus NCFM®, which was followed up by a second assessment of stool tests and questionnaires taken 4-6 weeks after taking the probiotic. The participants saw a 73% improvement in bloating and 62% improvement in pain and cramps (Faber, SM. 2000).
In a 2007 study, a potential mechanism of action for this pain-relieving effect was determined. Lactobacillus acidophilus NCFM® was shown to induce expression of μ-opioid and cannabinoid receptors in intestinal epithelial cells, leading to an analgesic effect similar to that of the pain-killer morphine, and resulting in a measurable reduction of colonic pain (Rousseaux, C et al., 2007).
More recent studies appear to have confirmed this mechanism of action. It has been identified that the bacteria actually communicate with cells in the gut wall and help to modulate the signals which are involved in creating spasms, thereby alleviating cramps and associated pain.
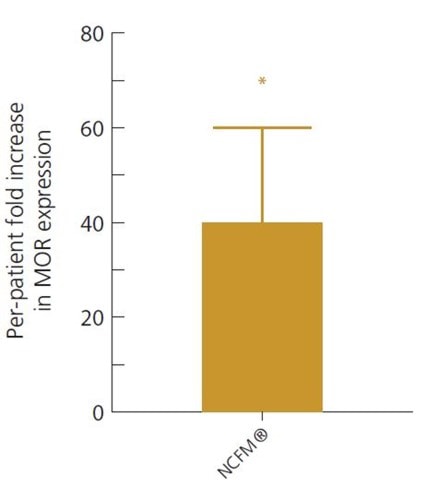
This was again demonstrated in a double-blind, randomised, controlled clinical trial, where 20 IBS sufferers took either Lactobacillus acidophilus NCFM® alone or a combination of L. acidophilus NCFM® and Bifidobacterium lactis Bi-07® twice a day for 3 weeks. The cell-signalling pathways involved in IBS symptoms, such as the stimulation of the opioid receptors, were looked at by examining samples of gut tissue from each participant. Subjects in both groups reported an improvement in IBS symptoms, but it was noted that only those supplemented with the single strain of Lactobacillus acidophilus NCFM® had a significant decrease in levels of the pain perception signalling pathways in their tissue samples, suggesting that this strain has specific potential to modulate mu-opioid receptor (MOR) expression and activity.
Intestinal tissue samples showed L. acidophilus NCFM® is able to reduce pain in the gut by increasing MOR receptor expression 40-fold, when compared to pre-study tissue samples (Ringel-Kulka, 2014).
This beneficial effect has been further explored recently, in a large triple blind, placebo controlled trial which included 391 subjects suffering from IBS-related pain. In this trial, participants were given either a supplement containing 1 billion CFU or 10 billion CFU of Lactobacillus acidophilus NCFM® every day for a period of 12 weeks, or a placebo.
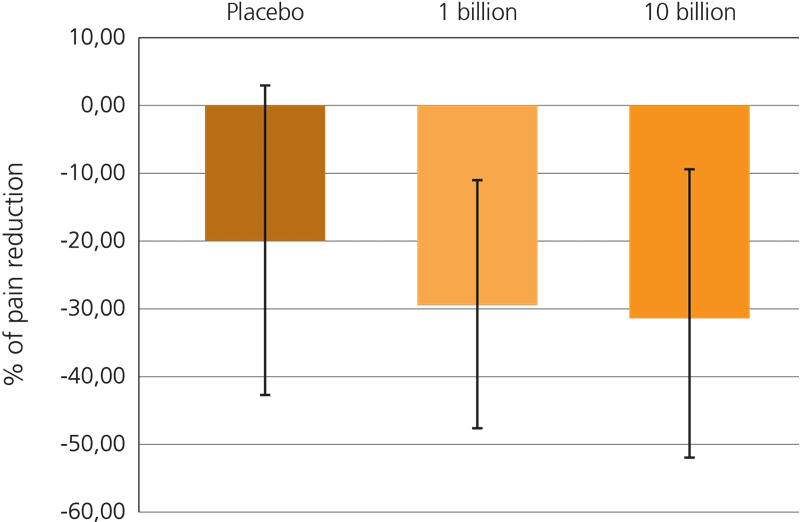
Both probiotic groups specifically showed a reduction in gut pain, but it was noted that the higher dose of live cultures yielded a more statistically significant result: Lactobacillus acidophilus NCFM® at a dose of 10 billion CFU reduced discomfort in the gut by over 30% from the baseline. This trial is particularly relevant for sufferers of IBS who frequently experience spasms, cramps and abdominal pain (Lyra A. et al, 2016).
Further relevant studies: D’Souza et al. (2015), Faber (2003), Hong & Rhee (2014), Ringel, Y. et al. (2008), Ringel-Kulka et al. (2011).
Diarrhoea is a disturbing digestive symptom that can be triggered by a variety of causes including bacterial infection, viral infection or Irritable Bowel Syndrome (IBS). Studies have shown that Lactobacillus acidophilus NCFM® may help to alleviate this symptom,
In a double-blind, placebo-controlled, randomised clinical study involving 243 children aged 12-36 months, Lactobacillus acidophilus NCFM® was given to subjects as part of a three strain formulation (also including Bifidobacterium lactis Bi-07®). During the 14-week trial, a statistically significant reduction in the incidence and frequency of episodes of diarrhoea was seen in the probiotic group versus the placebo (Ruiz-Palacios et al, 1996).
In another study using HIV-positive subjects with diarrhoea, it was found that a combination of L. acidophilus NCFM®, Bifidobacterium lactis Bi-07®, and soluble fibre significantly reduced the frequency and number of stools passed. The subjects had previously been using loperamide (also known as Imodium) to help manage their symptoms, but during the study found that they also needed to use less of the medication (Heiser et al, 2004).
Constipation is a widespread digestive disorder, particularly in western countries and so, as you will see from other pages in the database, the use of probiotics to help manage this symptom has been the focus of a growing area of research. Lactobacillus acidophilus NCFM® is one of the bacterial strains that has yielded encouraging results in for the alleviation of slow stool transit.
A recent study investigated this potential and yielded positive results. Subjects with constipation were randomly divided into two groups and given a yoghurt each morning for 14 days. The members of the treatment group were given a yoghurt containing a combination of a soluble fibre with the probiotic strains Lactobacillus acidophilus NCFM® and Bifidobacterium lactis HN019, and those in the control group were given a plain yoghurt.
Results indicated that those in the treatment group showed a significantly shortened colon transit time after two weeks (Magro et al., 2014).
Further relevant studies: Waitzberg et al (2013).
Lactose intolerance is one of the most common food intolerances, caused by an inability to digest lactose, a type of sugar found primarily in milk and dairy products. This intolerance results from a deficit of the enzyme lactase, needed in the body to efficiently break down lactose into simpler forms which can then be more easily absorbed into the bloodstream. Lactobacillus bacteria possess lactase activity and so may help to aid digestion of lactose.
There has been a small amount of research into the particular potential of Lactobacillus acidophilus NCFM® to relieve the symptoms of lactose intolerance. In a small study from 1995, nine out of ten lactose-intolerant subjects experienced a reduction in symptoms following ingestion of milk which had been inoculated with Lactobacillus acidophilus NCFM®, compared to when they consumed un-inoculated milk (Montes et al., 1995).
Emerging research suggests a role for the gastro-intestinal microbiota as a vital part of the immune response in humans and all other vertebrates. Lactobacillus acidophilus NCFM® is one of the strains that has been studied to determine its role in immune function.
A study showed positive effects of Lactobacillus acidophilus NCFM®, on serum immunoglobulins in healthy adults following vaccinations, with an increase in levels of serum IgA and IgM. Immunoglobulin A (IgA, also referred to as sIgA) is an antibody known to play a vital role in the immune response of mucous membranes, and Immunoglobulin M, or IgM, is the first antibody to be stimulated on initial exposure to an antigen. Although the precise mechanism by which the bacteria may affect these immunoglobulins is not yet fully understood, this is a strong indication that this strain can modulate, or have a positive effect on our immune responses (Paineau, D. et al., 2008).
These positive results have prompted further studies into the effects of probiotics on immune function, and Lactobacillus acidophilus NCFM® has also been studied in combination with other strains. In one double-blind, placebo-controlled study, 326 eligible children from 3 to 5 years of age were randomised to receive placebo, Lactobacillus acidophilus NCFM®, or Lactobacillus acidophilus NCFM® in combination with Bifidobacterium lactis Bi-07®. The children were given the supplement twice each day for a period of 6 months.
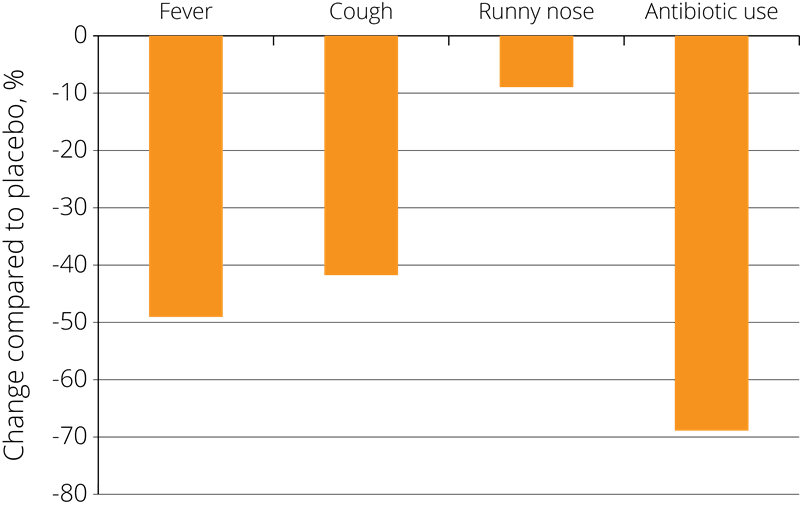
The results showed a statistically significant reduction in the incidence of cold symptoms such as fevers, coughs, and rhinorrhoea (runny nose) and associated use of antibiotics in both of the probiotic groups compared to the placebo group, resulting in fewer days absent from school due to illness - see right (Leyer G.J., et al. 2009).
In another randomised trial of 47 children with hay fever (triggered by birch tree pollen), the effects of the same probiotic combination on allergy symptoms were assessed. The children were either given a supplement containing Lactobacillus acidophilus NCFM® in combination with Bifidobacterium lactis Bl-04®, or a placebo. In peak hay fever season, tests revealed that those taking the two strain probiotic combination showed a significantly reduced presence of eosinophils (the white blood cells involved in controlling allergies) in the nasal area, compared to the placebo group. It may be important to note that all the participants were also given oral antihistamines to ease their symptoms. This undoubtedly reduced the possibility of properly observing a treatment effect on all pollen allergy symptoms. (Ouewhand, AC et al., 2009).
Further Relevant Studies: Bjorklund et al., (2011), Cai, M.et al., (2008), Foligne et al., (2007a), Foligne et al., (2007b), Konstantinov et al., (2008), Leyer et al., (2009), Ouwehand A., (2008), West, (2014), Zoumpopoulou et al., (2009).
Antibiotics are medications used to treat bacterial infections; they are routinely used world-wide to treat a very broad range of diseases and infections including respiratory tract infections, urinary tract infections, skin infections and infected wounds. Though antibiotics often have life-saving effects, they can also have a range of unpleasant side effects including antibiotic-associated diarrhoea.
Antibiotics can have an indiscriminate effect, killing strains of beneficial bacteria in the body as well as those causing the infection. It can take the resident flora a long time to rebalance after antibiotic treatment because our bodily populations of beneficial bacteria form one of our defences against pathogenic strains. Antibiotic treatment may leave the body even more vulnerable to infection once the course of medication is completed (Engelbrekston AL et al 2009).
Scientists are therefore exploring ways of quickly restoring the resident microbiota after antibiotic treatment, and have focused on the use of probiotics to help minimise the side effects of antibiotics. Lactobacillus acidophilus NCFM® is one of the strains that has been studied for this purpose.
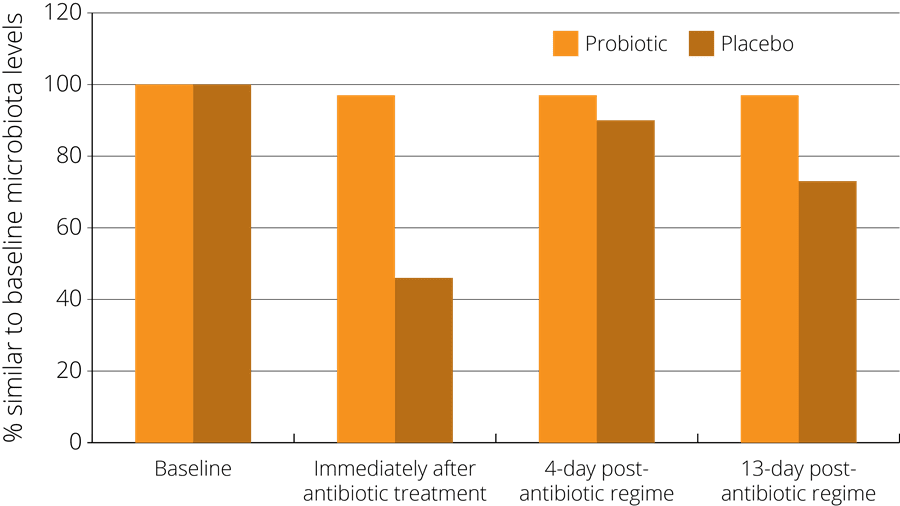
In one such study, a probiotic supplement containing a combination of strains including Lactobacillus acidophilus NCFM® was found to minimise the disturbance of faecal microbiota after antibiotic treatment. The study observed 51 healthy subjects who had been administered with antibiotics, and randomised to either receive a probiotic or a placebo at the same time. The subjects gave faecal samples both before and after the treatment in order to compare their gut microbiota at both stages. The results indicated that those subjects in the probiotic group showed less of a disturbance in their faecal microbiota, which then returned more rapidly to the pre-antibiotic baseline state - see right (Engelbrektson, A.L. et al., 2009).
In another randomised, double-blind, placebo-controlled, parallel group study, Lactobacillus acidophilus NCFM® was given in combination Bifidobacterium lactis Bi-07® to 111 healthy subjects, during and after antibiotic treatment.
The faecal microbiota was monitored by real time-polymerase chain react to assess the effects of the probiotics, and it was found that the consumption of the probiotic combination led to an increase in the faecal levels of the probiotic species. The reduction in Lactobacilli species that was observed in the placebo group was not noted in the probiotic treatment groups, and it was thought that consuming a probiotic containing a Lactobacillus strain may have helped to stabilise Lactobacillus populations in the gut (Forssten et al., 2014).
Further relevant studies: Ouwehand et al (2014), Schrezenmeir et al (2004), Engelbrekston et al (2006)
There is a growing level of interest in the use of probiotics to help address the overgrowth of pathogenic yeasts, typically caused by the Candida family. These yeasts are natural residents in the human body, and are typically kept in check by the indigenous microbiome; however, they are opportunistic pathogens which may proliferate and cause fungal infections if conditions are favourable. To date, there has been a limited amount of research in this area, but Lactobacillus acidophilus NCFM® is one of the bacterial strains that has been studied for its effects against pathogens such as Candida albicans. Due to its antimicrobial activity against common intestinal pathogens and their toxins, it is thought that Lactobacillus acidophilus NCFM® may improve the composition of the intestinal microbiota. This may lead to a reduced risk of intestinal disorders and provide a protective effect against systemic infections caused by pathogens such as Candida albicans and Citrobacter.
The research has mostly been in the form of murine (mice) studies. One such study looked at how prior colonisation with Lactobacillus acidophilus NCFM® and other probiotic bacteria affected the antibody responses of immune-deficient mice. The study compared the results with the antibody responses produced by mice with an alimentary tract colonised only with Candida albicans. It was demonstrated that, although the probiotic bacteria did not appear to induce a strong antibody response to their own antigens, they altered the rodents’ antibody response against Candida albicans (Wagner et al., 2000a).
Other relevant studies: Chen et al (2009), Lahtinen, S.J et al (2012), Wagner et al (2000b). Wagner et al (1998).
Eating disorders after bariatric surgery are difficult to assess and may be under-reported.
The use of probiotics as a facilitator in the treatment of eating disorders has already been investigated as a means of modulating the microbiota-gut-brain axis.
In a randomised, double-blind placebo-controlled trial, 101 patients received either probiotic supplementation of Lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 or placebo for 90 days after bariatric surgery, starting on the seventh postoperative day. The Yale Food Addiction Scale (YFAS) and Binge Eating Scale (BES) were applied to assess food addiction and binge eating, respectively.
Before surgery, one-third of the patients presented with a food addiction and binge eating diagnosis. After surgery a significant effect of treatment with probiotics was observed 1 year on. Both the number of symptoms of food addiction and the binge eating score were lower in the probiotic group than in the placebo group (p=0.037 and p=0.030, respectively). (Ramos et al., 2022)
Authors: Information on this strain was gathered by Joanna Scott-Lutyens BA (hons), DipION, Nutritional Therapist; and Kerry Beeson, BSc (Nut.Med) Nutritional Therapist.
Last updated - 12th May 2020
As some properties & benefits of probiotics may be strain-specific, this database provides even more detailed information at strain level. Read more about the strains that we have included from this genus below.
Lactobacillus acidophilus strains: Lactobacillus acidophilus LA-05, Lactobacillus acidophilus Rosell-52.
Lactobacillus casei strains: Lactobacillus casei Shirota, Lactobacillus casei DN-114001.
Lactobacillus plantarum strains: Lactobacillus plantarum LP299v.
Lactobacillus reuteri strains: Lactobacillus reuteri Protectis and Lactobacillus reuteri RC-14®.
Lactobacillus rhamnosus strains: Lactobacillus rhamnosus LGG®, Lactobacillus rhamnosus HN001, Lactobacillus rhamnosus GR-1® and Lactobacillus rhamnosus Rosell-11.
Lactobacillus paracasei strains: Lactobacillus paracasei CASEI 431®.
For products containing this strain visit the Optibac Probiotics shop.
For more info and the latest research on probiotics, please visit the Probiotic Professionals pages.
Altermann, E. et al., (2005). ‘Complete genome sequence of the probiotic lactic acid bacterium Lactobacillus acidophilus NCFM’, Proceedings of the National Academy of Sciences of the United States of America, 102(11):3906–3912.
Andreasen, A.S. et al., (2010). ‘Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects’. Br J Nutr 104: 1831-1838.
Azcarate-Peril, MA et al., (2004). ‘Identification and inactivation of genetic loci involved with Lactobacillus acidophilus acid tolerance’. Appl. Environ. Microbiol., 70(9):5315-22.
Azcarate-Peril, M. A. et al., (2005). ‘Microarray analysis of a two-component regulatory system involved in acid resistance and proteolytic activity in Lactobacillus acidophilus’. Appl. Environ. Microbiol. 71:5794-5804.
Azcarate-Peril, M.A. et al., (2006). ‘Transcriptional and functional analysis of oxalyl-coenzyme A (CoA) decarboxylase and formyl-CoA transferase genes from Lactobacillus acidophilus’. Appl Environ Microbiol., 72(3):1891-1899.
Azcarate-Peril, M. A et al., (2009). ‘Temporal gene expression and probiotic attributes of Lactobacillus acidophilus during growth in milk’. J. Dairy Sci., 92:870-88.
Barefoot, S. F. and Klaenhammer, T. R. (1984). ‘Purification and characterization of the Lactobacillus acidophilus bacteriocin lactacin B’. Antimicrob. Agents Chemotherapy, 26:328- 334.
Barefoot, S. F. and Klaenhammer, T. R. (1983). ‘Detection and activity of lactacin B, a bacteriocin produced by Lactobacillus acidophilus’. Appl. Environ. Microbiol. 45:1808-1815.
Barrangou, R. et al., (2003). ‘Functional and comparative genomic analyses of an operon involved in fructooligosaccharide utilization by Lactobacillus acidophilus’. Proc Natl Acad Sci USA., 100:8957-8962.
Barrangou, R., et al., (2006). ‘Global analysis of carbohydrate utilization by Lactobacillus acidophilus by cDNA microarrays’. Proc Natl Acad Sci., USA 103: 3816-3821.
Bjorklund, M., et al., (2011). ‘Gut microbiota of healthy elderly NSAID users is selectively modified with the administration of Lactobacillus acidophilus NCFM and lactitol’. Age (Dordr)., 34(4): 987–999.
Buck, B.L. et al., (2009). ‘Role of autoinducer-2 on the adhesion ability of Lactobacillus acidophilus’ J. Appl. Microbiol., 107 (1): 269-279.
Buck, B.L. et al., (2005). ‘Functional Analysis of Putative Adhesion Factors in Lactobacillus acidophilus NCFM®’ Applied and Environmental Microbiology, 71 (12):8344–8351.
Bull, M., et al., (2013). ‘The life history of Lactobacillus acidophilus as a probiotic: a tale of revisionary taxonomy, misidentification and commercial success’. FEMS Microbiol Lett., 349:77–87.
Bull, M.J., et al., (2014). ‘The domestication of the probiotic bacterium Lactobacillus acidophilus’. Sci. Rep., 4:7202.
Cai, M., et al., (2008). ‘Animal studies on enhancement of immune function by dietary probiotic supplementation of Lactobacillus acidophilus NCFM® and Bifidobacterium lactis Bi-07’. Chinese Journal of Microecology, 20(1):17-19.
Calder, P.C. (2013). ‘Feeding the immune system’. Proc Nutr Soc., 72(3):299-309.
Chen, C.C., et al., (2009). ‘Effect of probiotics L. acidophilus on C. rodentium colitis: the role of dendritic cells’. Paediatric Research, 65 (2):169-175.
Collado, M.C., et al., (2007), ‘Role of commercial probiotic strains against human pathogen adhesion to intestinal mucus’. Letters in Applied Microbiology, 45:454–460.
Collado, M.C., et al., (2008). ’Adhesion and aggregation properties of probiotic and pathogen strains’. Eur Food Res Technol., 226(5):1065-1073.
Crowell, D.C. (1998). ‘Microbial analysis of human intestinal flora after feeding Lactobacillus acidophilus’. Journal of Dairy Science, 84(2) 319–331.
Daniel, C., et al., (2006). ‘Selecting Lactic Acid Bacteria for Their Safety and Functionality by Use of a Mouse Colitis Model’. Applied and Environmental Microbiology, 72(9):5799–5805.
D’Souza, B., et al. (2015), ‘Randomized controlled trial of probiotics after colonoscopy’. ANZ Journal of Surgery, doi: 10.1111/ans.13225.
Engelbrektson, A.L .et al., (2009). ‘Probiotics to minimize the disruption of faecal microbiota in healthy subjects undergoing antibiotic therapy’. Journal of Medical Microbiology, 58:663-670.
Engelbrektson, A.L. et al., (2006). ‘Analysis of treatment effects on the microbial ecology of the gastrointestinal tract’. FEMS Microbiol. Ecol. 57:239-250.
Faber S.E., (2003), ‘Comparison of probiotics with antibiotics to probiotics alone in treatment of diarrhea predominant IBS (D-IBS), alternating (A-IBS) and constipation (C-IBS) patients’, Gastroenterology, 124(4):A687-A688.
Faber, S.M., (2000). ‘Treatment of abnormal gut flora improves symptoms in patients with irritable bowel syndrome’. American Journal of Gastroenterology, 95(9):2533.
Forssten, S., et al., (2014). ‘Influence of a probiotic mixture on antibiotic induced microbiota disturbances’. World Journal of Gastroenterology, 20(33):11878-85.
Foligne, B., et al., (2007). ‘Correlation between in vitro and in vivo immunomodulatory properties of lactic acid bacteria’. World Journal of Gastroenterology, 13(2):236-243.
Foligne, B., et al., (2007). ‘A Key Role of Dendritic Cells in Probiotic Functionality’. PloS ONE 2(3): e313.
Fotiadis, C. I. et al., (2008) ‘Role of probiotics, prebiotics and synbiotics in chemoprevention for colorectal cancer’. World Journal of Gastroenterology, 14(42):6453-6457.
Gilliland, S.W. et al., (1978) ‘Influence of consuming non fermented milk containing Lactobacillus acidophilus on fecal flora of healthy males’. Journal of Dairy Science, 61:1-10.
Goldin, B.R. and Gorbach, S.L. (1984). ‘The effect of milk and Lactobacillus feeding on human intestinal bacterial enzyme activity’. American Journal of Clinical Nutrition, 39:756-761.
Heiser, C.R. et al. (2004). ‘Probiotics, soluble fiber, and L Glutamine (GLN) reduce nelfinavir (NFV) - or lopinavir/ritonavir (LPV/r)-related diarrhea.’ J. Int. Assoc Physicians AIDS Care (Chic Ill), 3(4):121-129.
Hong, S.N. and Rhee, P.L. (2014). ‘Unravelling the ties between irritable bowel syndrome and intestinal microbiota’. World Journal of Gastroenterology, 20(10):2470-2481.
Kim, H.S. and Gilliland, S.E. (1983). ‘Lactobacillus acidophilus as a dietary adjunct for milk to aid lactose digestion in humans’. J. Dairy Science, 66(5):959-966.
Konstantinov, S.R., et al., (2008). ‘S layer protein A of Lactobacillus acidophilus NCFMR regulates immature dendritic cell and T cell functions’. Proceedings of the National Academy of Science, 105(49):19474-9.
Lahtinen, S.J. et al., (2012). ‘Probiotic cheese containing Lactobacillus rhamnosus HN001 and Lactobacillus acidophilus NCFM® modifies subpopulations of fecal lactobacilli and Clostridium difficile in the elderly’. Age (Dordr). 34(1):133-43.
Leyer, G.J., et al., (2009). ‘Probiotic Effects on Cold and Influenza-Like Symptom Incidence and Duration in Children’. Pediatrics; 124 (2): 172-179.
Lyra, A. et al., (2016) ‘Irritable bowel syndrome symptom severity improves equally with probiotic and placebo’. World Journal of Gastroenterology. 22 (48):10631–10642.
Magro, D.O., et al., (2014). ‘Effect of yogurt containing polydextrose, Lactobacillus acidophilus NCFM and Bifidobacterium lactis HN019: a randomized, double-blind, controlled study in chronic constipation’. Nutrition Journal, 13:75.
Mai, V., et al. (2017) ‘Novel encapsulation improves recovery of probiotic strains in fecal samples of human volunteers’. Appl Microbiol Biotechnol, 101(4):1419-1425.
McAuliffe, O., Cano, R.J. and Klaenhammer, T.R. (2005). ‘Genetic analysis of two bile salt hydrolase activities in Lactobacillus acidophilus NCFM®’. Appl Environ Microbiol., 71(8):4925-4929.
Morovic, W., et al. (2017) ‘Safety evaluation of HOWARU® Restore (Lactobacillus acidophilus NCFM®, Lactobacillus paracasei Lpc-37, Bifidobacterium animalis subsp. lactis Bl-04 and B. lactis Bi-07) for antibiotic resistance, genomic risk factors, and acute toxicity’. Food Chem Toxicol, 110:316-324.
Montes, R.G. et al., (1995). ‘Effect of Milks Inoculated with Lactobacillus acidophilus or a Yogurt Starter Culture in Lactose-Maldigesting Children’. Journal of Dairy Science. 78(8): 1657 – 1664.
Mustapha A. (1997). ‘Improvement of lactose digestion by humans following ingestion of unfermented acidophilus milk: influence of bile sensitivity, lactose transport, and acid tolerance of Lactobacillus acidophilus’. J Dairy Sci., 80, 8. 1537-45.
Ouwehand, A.C. et al., (2009). ‘Influence of a combination of Lactobacillus acidophilus NCFM® and lactitol on healthy elderly: intestinal and immune parameters’. British Journal of Nutrition. 101(3):367-75.
Ouewhand, A.C. et al., (2009). ‘Specific probiotics alleviate allergic rhinitis during the birch pollen season’. World Journal of Gastronenterology. 15(26): 3261-3268.
Ouwehand, A.C., et al., (2014). ‘Probiotics reduce symptoms of antibiotic use in a hospital setting: a randomized dose response study’. Vaccine.32 (4):458-63.
Ouwehand A. Leyer G., and Carcano D., (2008). ‘Probiotics reduce incidence and duration of respiratory tract infection symptoms in 3 to 5-year-old children’. Pediatrics. 121(1):115.
Ouwehand, A.C. and Lahtinen, S., (2009). ‘Lactobacillus acidophilus NCFMR. In: Handbook of probiotics and prebiotics’. Lee, Y.K. & Salminen, S. (eds.) John Wiley & Sons.
Paineau D. et al., (2008). ‘Effects of seven potential probiotic strains on specific immune responses in healthy adults: a double-blind, randomised, controlled trial’. FEMS Immunology and Medical Microbiology, 53(1):107-13.
Ramos, M.R.Z. et al., (2022). 'Probiotic Supplementation Attenuates Binge Eating and Food Addiction 1 Year After Roux-en-Y Gastric Bypass: A Randomized, Double-Blind, Placebo-Controlled Trial'. Brazilian Archives of Digestive Surgery, 35:e1659.
Rao C. et al., (1999). ‘Prevention of colonic preneoplastic lesions by the probiotic Lactobacillus acidophilus NCFM® in F344 rats’. Int. Journal of Oncology. 14:939-944.
Reid G., (2000). ‘Investigation of the properties of Lactobacillus acidophilus NCFM™ as a possible probiotic for the urogenital tract’. International Dairy Journal 10, (5-6): 415-419.
Ringel-Kulka T. et al., (2011). ‘Probiotic Bacteria Lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 Versus Placebo for the Symptoms of Bloating in Patients with Functional Bowel Disorders:a Double-blind Study’. J Clin Gastroenterol., 45: 518-525.
Ringel-Kulka (2014). ‘Lactobacillus acidophilus NCFM® affects colonic mucosal opioid receptor expression in patients with functional abdominal pain - a randomised clinical study’. Ailment. Pharmacological. Therapy, 40(2):200-7.
Rousseaux C. et al., (2007), ‘Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors’. Nature Medicine, 13(1):35-7.
Ringel Y. et al., (2008). ‘Probiotic bacteria Lactobacillus acidophilus NCFM® and Bifidobacterium lactis Bi-07 improve symptoms of bloating in patients with function bowel disorders (FBD)’. Gastoenterology; 134(4 Suppl.1):A549.
Ringel-Kulka T., et al., (2011). ‘Probiotic Bacteria Lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 Versus Placebo for the Symptoms of Bloating in Patients with Functional Bowel Disorders. A Double-blind Study’. Journal of Clinical Gastroenterology, 45: 518-525.
Ringel-Kulka T., et al., (2014). ‘Lactobacillus acidophilus NCFM affects colonic mucosal opioid receptor expression in patients with functional abdominal pain – a randomised clinical study’. Aliment Pharmacological Therapy., 40(2):200-7. doi: 10.1111/apt.12800.
Ruiz-Palacios G. et al., (1996). ‘Feeding of a probiotic for the prevention of community acquired diarrhea in young Mexican children’. Pediatric Research, 39 (Pt. 2):104, Abstr. #1089.
Sanders M. E, and Klaenhammer, T. R., (2001). ‘Invited Review: The Scientific Basis of Lactobacillus acidophilus NCFM Functionality as a Probiotic’. Journal of Dairy Science Vol. 84(2):319-331.
Schrezenmeir J. et al., (2004). ‘Benefits of Oral Supplementation with and without Synbiotics in Young Children with Acute Bacterial Infections’. Clinical Pediatrics, 43;239-249.
Sui, J., Leighton S., Busta F. and Brady L. (2002). ‘16S ribosomal DNA analysis of the faecal lactobacilli composition of human subjects consuming a probiotic strain Lactobacillus acidophilus NCFM®’. Journal of Applied Microbiology, 93:907–912.